Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction
- PMID: 34269488
- PMCID: PMC8518397
- DOI: 10.1002/chem.202101880
Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction
Abstract
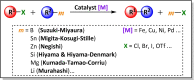
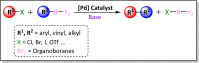
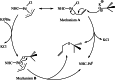
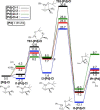
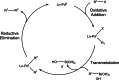
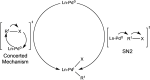
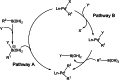
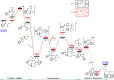
The story of C-C bond formation includes several reactions, and surely Suzuki-Miyaura is among the most outstanding ones. Herein, a brief historical overview of insights regarding the reaction mechanism is provided. In particular, the formation of the catalytically active species is probably the main concern, thus the preactivation is in competition with, or even assumes the role of the rate determining step (rds) of the overall reaction. Computational chemistry is key in identifying the rds and thus leading to milder conditions on an experimental level by means of predictive catalysis.
Keywords: Cross coupling; C−C bond formation; DFT; Suzuki-Miyaura; palladium; preactivation.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









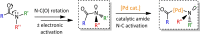
Similar articles
-
Palladium-catalyzed decarbonylative Suzuki-Miyaura cross-coupling of amides by carbon-nitrogen bond activation.Chem Sci. 2019 Sep 3;10(42):9865-9871. doi: 10.1039/c9sc03169c. eCollection 2019 Nov 14. Chem Sci. 2019. PMID: 32015810 Free PMC article.
-
Asymmetric Construction of an Aryl-Alkene Axis by Palladium-Catalyzed Suzuki-Miyaura Coupling Reaction.Angew Chem Int Ed Engl. 2022 Nov 7;61(45):e202211211. doi: 10.1002/anie.202211211. Epub 2022 Oct 6. Angew Chem Int Ed Engl. 2022. PMID: 36111538
-
Computationally Assisted Mechanistic Investigation and Development of Pd-Catalyzed Asymmetric Suzuki-Miyaura and Negishi Cross-Coupling Reactions for Tetra-ortho-Substituted Biaryl Synthesis.ACS Catal. 2018 Nov 2;8(11):10190-10209. doi: 10.1021/acscatal.8b02509. Epub 2018 Sep 20. ACS Catal. 2018. PMID: 30450265 Free PMC article.
-
Transmetalation in the Suzuki-Miyaura coupling: the fork in the trail.Angew Chem Int Ed Engl. 2013 Jul 15;52(29):7362-70. doi: 10.1002/anie.201301737. Epub 2013 Jun 18. Angew Chem Int Ed Engl. 2013. PMID: 23780626 Review.
-
Suzuki-Miyaura (hetero-)aryl cross-coupling: recent findings and recommendations.Chem Soc Rev. 2025 Jun 16;54(12):5746-5765. doi: 10.1039/d4cs01108b. Chem Soc Rev. 2025. PMID: 40392002 Review.
Cited by
-
Palladium(II) and Platinum(II) Complexes Bearing ONS-Type Pincer Ligands: Synthesis, Characterization and Catalytic Investigations.Molecules. 2024 Jul 22;29(14):3425. doi: 10.3390/molecules29143425. Molecules. 2024. PMID: 39065003 Free PMC article.
-
Synthesis of 2-arylpyridines by the Suzuki-Miyaura cross-coupling of PyFluor with hetero(aryl) boronic acids and esters.Can J Chem. 2023 Oct;101(10):765-772. doi: 10.1139/cjc-2023-0033. Epub 2023 Aug 30. Can J Chem. 2023. PMID: 38550267 Free PMC article.
-
Distinguishing Competing Mechanistic Manifolds for C(acyl)-N Functionalization by a Ni/N-Heterocyclic Carbene Catalyst System.JACS Au. 2023 Aug 21;3(9):2451-2457. doi: 10.1021/jacsau.3c00283. eCollection 2023 Sep 25. JACS Au. 2023. PMID: 37772178 Free PMC article.
-
Characterization of the 1-(5-(4,5-Dimethyl-1,3,2-dioxoborolan-2-yl)thiophen-2-yl)ethanone Using NMR 13C, 1H and 11B through the Density Functional Theory.Materials (Basel). 2023 Apr 12;16(8):3037. doi: 10.3390/ma16083037. Materials (Basel). 2023. PMID: 37109875 Free PMC article.
-
Optoelectronic Response to the Fluor Ion Bond on 4-(4,4,5,5-Tetramethyl-1,3,2-dioxoborolan-2-yl)benzaldehyde.Int J Mol Sci. 2024 May 3;25(9):5000. doi: 10.3390/ijms25095000. Int J Mol Sci. 2024. PMID: 38732218 Free PMC article.
References
-
- Beletskaya I. P., Alonso F., Tyurin V., Coord. Chem. Rev. 2019, 385, 137–173.
-
- Johansson Seechurn C. C. C., Kitching M. O., Colacot T. J., Snieckus V., Angew. Chem. Int. Ed. 2012, 51, 5062–5085. - PubMed
-
- Negishi E., Bull. Chem. Soc. Jpn. 2007, 80, 233–257.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

