Decision Making When Cancer Becomes Chronic: Needs Assessment for a Web-Based Medullary Thyroid Carcinoma Patient Decision Aid
- PMID: 34269691
- PMCID: PMC8325074
- DOI: 10.2196/27484
Decision Making When Cancer Becomes Chronic: Needs Assessment for a Web-Based Medullary Thyroid Carcinoma Patient Decision Aid
Abstract
Background: In cancers with a chronic phase, patients and family caregivers face difficult decisions such as whether to start a novel therapy, whether to enroll in a clinical trial, and when to stop treatment. These decisions are complex, require an understanding of uncertainty, and necessitate the consideration of patients' informed preferences. For some cancers, such as medullary thyroid carcinoma, these decisions may also involve significant out-of-pocket costs and effects on family members. Providers have expressed a need for web-based interventions that can be delivered between consultations to provide education and prepare patients and families to discuss these decisions. To ensure that these tools are effective, usable, and understandable, studies are needed to identify patients', families', and providers' decision-making needs and optimal design strategies for a web-based patient decision aid.
Objective: Following the international guidelines for the development of a web-based patient decision aid, the objectives of this study are to engage potential users to guide development; review the existing literature and available tools; assess users' decision-making experiences, needs, and design recommendations; and identify shared decision-making approaches to address each need.
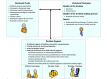
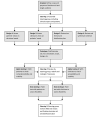
Methods: This study used the decisional needs assessment approach, which included creating a stakeholder advisory panel, mapping decision pathways, conducting an environmental scan of existing materials, and administering a decisional needs assessment questionnaire. Thematic analyses identified current decision-making pathways, unmet decision-making needs, and decision support strategies for meeting each need.
Results: The stakeholders reported wide heterogeneity in decision timing and pathways. Relevant existing materials included 2 systematic reviews, 9 additional papers, and multiple educational websites, but none of these met the criteria for a patient decision aid. Patients and family members (n=54) emphasized the need for plain language (46/54, 85%), shared decision making (45/54, 83%), and help with family discussions (39/54, 72%). Additional needs included information about uncertainty, lived experience, and costs. Providers (n=10) reported needing interventions that address misinformation (9/10, 90%), foster realistic expectations (9/10, 90%), and address mistrust in clinical trials (5/10, 50%). Additional needs included provider tools that support shared decision making. Both groups recommended designing a web-based patient decision aid that can be tailored to (64/64, 100%) and delivered on a hospital website (53/64, 83%), focuses on quality of life (45/64, 70%), and provides step-by-step guidance (43/64, 67%). The study team identified best practices to meet each need, which are presented in the proposed decision support design guide.
Conclusions: Patients, families, and providers report multifaceted decision support needs during the chronic phase of cancer. Web-based patient decision aids that provide tailored support over time and explicitly address uncertainty, quality of life, realistic expectations, and effects on families are needed.
Keywords: clinical trial; decision support techniques; medullary thyroid cancer; mobile phone; oncology; patient decision aids; targeted therapy.
©Danielle Shojaie, Aubri S Hoffman, Ruth Amaku, Maria E Cabanillas, Julie Ann Sosa, Steven G Waguespack, Mark E Zafereo, Mimi I Hu, Elizabeth E Grubbs. Originally published in JMIR Formative Research (https://formative.jmir.org), 16.07.2021.
Conflict of interest statement
Conflicts of Interest: JAS is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry and is supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly. She receives institutional research funding from Exelixis and Eli Lilly. MEC is part of the advisory board in Exelixis. All other authors declare that they do not have any real or perceived conflicts of interest.
Figures

Similar articles
-
Patients' and Providers' Needs and Preferences When Considering Fertility Preservation Before Cancer Treatment: Decision-Making Needs Assessment.JMIR Form Res. 2021 Jun 7;5(6):e25083. doi: 10.2196/25083. JMIR Form Res. 2021. PMID: 34096871 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Palliative care experiences of adult cancer patients from ethnocultural groups: a qualitative systematic review protocol.JBI Database System Rev Implement Rep. 2015 Jan;13(1):99-111. doi: 10.11124/jbisrir-2015-1809. JBI Database System Rev Implement Rep. 2015. PMID: 26447011
-
An Online Decision Aid to Help Patients and Caregivers Decide on Treatments for Sickle Cell Disease [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Jan. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Jan. PMID: 39361785 Free Books & Documents. Review.
-
What factors hinder the decision-making process for women with cancer and contemplating fertility preservation treatment?Hum Reprod Update. 2017 Jul 1;23(4):433-457. doi: 10.1093/humupd/dmx009. Hum Reprod Update. 2017. PMID: 28510760 Review.
Cited by
-
Impact of Psychological Resilience on the Fear of Pain and Activity Recovery in Postsurgical Patients: Observational Cohort Study.JMIR Form Res. 2025 Feb 7;9:e63556. doi: 10.2196/63556. JMIR Form Res. 2025. PMID: 39924300 Free PMC article.
-
What do patients want to know about surgery for low-risk thyroid cancer? A qualitative study.Surgery. 2023 Jan;173(1):226-231. doi: 10.1016/j.surg.2022.05.049. Epub 2022 Nov 3. Surgery. 2023. PMID: 36336505 Free PMC article.
-
Limited Thyroidectomy Achieves Equivalent Survival to Total Thyroidectomy for Early Localized Medullary Thyroid Cancer.Cancers (Basel). 2024 Dec 4;16(23):4062. doi: 10.3390/cancers16234062. Cancers (Basel). 2024. PMID: 39682246 Free PMC article.
-
Challenges in the care of patients with RET-altered thyroid cancer: a multicountry mixed-methods study.Thyroid Res. 2023 Aug 14;16(1):22. doi: 10.1186/s13044-023-00166-4. Thyroid Res. 2023. PMID: 37574538 Free PMC article.
-
Patient and public involvement in cancer research: A scoping review.Cancer Med. 2023 Jul;12(14):15530-15543. doi: 10.1002/cam4.6200. Epub 2023 Jun 16. Cancer Med. 2023. PMID: 37329180 Free PMC article.
References
-
- Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG, American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015 Jun;25(6):567–610. doi: 10.1089/thy.2014.0335. http://europepmc.org/abstract/MED/25810047 - DOI - PMC - PubMed
-
- Wells SA, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, Baudin E, Elisei R, Jarzab B, Vasselli JR, Read J, Langmuir P, Ryan AJ, Schlumberger MJ. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012 Jan 10;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. http://europepmc.org/abstract/MED/22025146 - DOI - PMC - PubMed
-
- Elisei R, Schlumberger MJ, Müller SP, Schöffski P, Brose MS, Shah MH, Licitra L, Jarzab B, Medvedev V, Kreissl MC, Niederle B, Cohen EE, Wirth LJ, Ali H, Hessel C, Yaron Y, Ball D, Nelkin B, Sherman SI. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013 Oct 10;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. http://europepmc.org/abstract/MED/24002501 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

