Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE
- PMID: 34269818
- PMCID: PMC8796656
- DOI: 10.1182/blood.2020010439
Sustained minimal residual disease negativity in newly diagnosed multiple myeloma and the impact of daratumumab in MAIA and ALCYONE
Abstract
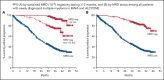
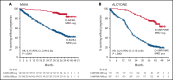
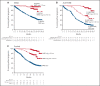
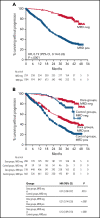
In patients with transplant-ineligible newly diagnosed multiple myeloma (NDMM), daratumumab reduced the risk of disease progression or death by 44% in MAIA (daratumumab/lenalidomide/dexamethasone [D-Rd]) and 58% in ALCYONE (daratumumab/bortezomib/melphalan/prednisone [D-VMP]). Minimal residual disease (MRD) is a sensitive measure of disease and response to therapy. MRD-negativity status and durability were assessed in MAIA and ALCYONE. MRD assessments using next-generation sequencing (10-5) occurred for patients achieving complete response (CR) or better and after at least CR at 12, 18, 24, and 30 months from the first dose. Progression-free survival (PFS) by MRD status and sustained MRD negativity lasting ≥6 and ≥12 months were analyzed in the intent-to-treat population and among patients achieving at least CR. In MAIA (D-Rd, n = 368; lenalidomide and dexamethasone [Rd], n = 369) and ALCYONE (D-VMP, n = 350; bortezomib/melphalan/prednisone [VMP], n = 356), the median duration of follow-up was 36.4 and 40.1 months, respectively. MRD-negative status and sustained MRD negativity lasting ≥6 and ≥12 months were associated with improved PFS, regardless of treatment group. However, daratumumab-based therapy improved rates of MRD negativity lasting ≥6 months (D-Rd, 14.9% vs Rd, 4.3%; D-VMP, 15.7% vs VMP, 4.5%) and ≥12 months (D-Rd, 10.9% vs Rd, 2.4%; D-VMP, 14.0% vs VMP, 2.8%), both of which translated to improved PFS vs control groups. In a pooled analysis, patients who were MRD negative had improved PFS vs patients who were MRD positive. Patients with NDMM who achieved MRD-negative status or sustained MRD negativity had deep remission and improved clinical outcomes. These trials were registered at www.clinicaltrials.gov as #NCT02252172 (MAIA) and #NCT02195479 (ALCYONE).
© 2022 by The American Society of Hematology.
Figures
Comment in
-
Sustained minimal residual disease in myeloma.Blood. 2022 Jan 27;139(4):469-471. doi: 10.1182/blood.2021013199. Blood. 2022. PMID: 35084473 No abstract available.
References
-
- Paiva B, Vidriales MB, Cerveró J, et al. ; GEM (Grupo Español de MM)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Groups . Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008;112(10):4017-4023. - PMC - PubMed
-
- Rawstron AC, Child JA, de Tute RM, et al. . Minimal residual disease assessed by multiparameter flow cytometry in multiple myeloma: impact on outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31(20):2540-2547. - PubMed
-
- Lahuerta JJ, Paiva B, Vidriales MB, et al. ; GEM (Grupo Español de Mieloma)/PETHEMA (Programa para el Estudio de la Terapéutica en Hemopatías Malignas) Cooperative Study Group . Depth of response in multiple myeloma: a pooled analysis of three PETHEMA/GEM clinical trials. J Clin Oncol. 2017;35(25):2900-2910. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

