L-type channel inactivation balances the increased peak calcium current due to absence of Rad in cardiomyocytes
- PMID: 34269819
- PMCID: PMC8289690
- DOI: 10.1085/jgp.202012854
L-type channel inactivation balances the increased peak calcium current due to absence of Rad in cardiomyocytes
Abstract
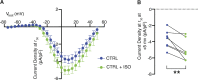
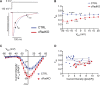
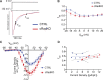
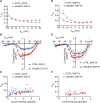
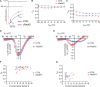
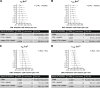
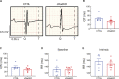
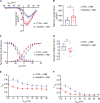
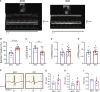
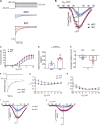
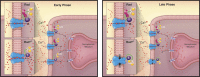
The L-type Ca2+ channel (LTCC) provides trigger calcium to initiate cardiac contraction in a graded fashion that is regulated by L-type calcium current (ICa,L) amplitude and kinetics. Inactivation of LTCC is controlled to fine-tune calcium flux and is governed by voltage-dependent inactivation (VDI) and calcium-dependent inactivation (CDI). Rad is a monomeric G protein that regulates ICa,L and has recently been shown to be critical to β-adrenergic receptor (β-AR) modulation of ICa,L. Our previous work showed that cardiomyocyte-specific Rad knockout (cRadKO) resulted in elevated systolic function, underpinned by an increase in peak ICa,L, but without pathological remodeling. Here, we sought to test whether Rad-depleted LTCC contributes to the fight-or-flight response independently of β-AR function, resulting in ICa,L kinetic modifications to homeostatically balance cardiomyocyte function. We recorded whole-cell ICa,L from ventricular cardiomyocytes from inducible cRadKO and control (CTRL) mice. The kinetics of ICa,L stimulated with isoproterenol in CTRL cardiomyocytes were indistinguishable from those of unstimulated cRadKO cardiomyocytes. CDI and VDI are both enhanced in cRadKO cardiomyocytes without differences in action potential duration or QT interval. To confirm that Rad loss modulates LTCC independently of β-AR stimulation, we crossed a β1,β2-AR double-knockout mouse with cRadKO, resulting in a Rad-inducible triple-knockout mouse. Deletion of Rad in cardiomyocytes that do not express β1,β2-AR still yielded modulated ICa,L and elevated basal heart function. Thus, in the absence of Rad, increased Ca2+ influx is homeostatically balanced by accelerated CDI and VDI. Our results indicate that the absence of Rad can modulate the LTCC without contribution of β1,β2-AR signaling and that Rad deletion supersedes β-AR signaling to the LTCC to enhance in vivo heart function.
© 2021 Ahern et al.
Figures






References
-
- Ahern, B.M., Levitan B.M., Veeranki S., Shah M., Ali N., Sebastian A., Su W., Gong M.C., Li J., Stelzer J.E., et al. . 2019. Myocardial-restricted ablation of the GTPase RAD results in a pro-adaptive heart response in mice. J. Biol. Chem. 294:10913–10927. 10.1074/jbc.RA119.008782 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

