Safety of COVID-19 vaccines
- PMID: 34270094
- PMCID: PMC8426829
- DOI: 10.1002/jmv.27214
Safety of COVID-19 vaccines
Abstract
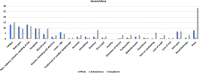
This study is aimed to identify the adverse effects associated with three types of coronavirus disease 2019 vaccines. Approximately 1736 individuals agreed to participate in this study. The participants involved in the study were individuals who had received the first dose or full course (two doses) of the vaccine at least 30 days before the survey. A direct and interactive web-based system interview with a paper and electronic version of the questionnaire was used for all participants. A total of 1736 randomized individuals were identified. The reactogenicity of the vaccines including pain, redness, urticaria, and swelling at the site of the injection was reported in 34.56% of the participants. Local site reaction was reported in more individuals who had Pfizer and AstraZeneca vaccines than those who received the Sinopharm vaccine. The systemic events were more common with AstraZeneca and Pfizer vaccines, symptoms reported were fatigue, body pain, headache, muscle pain, fever, and gastrointestinal side effects. There were no correlations between age or gender, and the duration of the adverse effects for the three vaccines. Swelling and severe allergic reaction of the eyelids, severe hypotension, generalized body aches, shortness of breath, weakness and numbness on the injected arm, acute hyperglycemia, severe chest pain, and fever more than 39°C were among the unusual signs and symptoms reported by the participants. Pfizer, AstraZeneca, and Sinopharm vaccines were found to be safe and Sinopharm vaccine showed a lower prevalence of adverse effects compared with the other vaccines. The duration and severity of adverse effects were not affected by age or gender. Unusual side effects should be closely monitored to establish determine they are linked to the immunization.
Keywords: AstraZeneca vaccine; COVID-19; Pfizer vaccine; Sinopharm vaccine; vaccine.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures



References
-
- Paltiel AD, Schwartz JL, Zheng A, Walensky RP. Clinical outcomes of a COVID‐19 vaccine: implementation over efficacy: study examines how definitions and thresholds of vaccine efficacy, coupled with different levels of implementation effectiveness and background epidemic severity, translate into outcomes. Health Aff. 2021;2020:02054‐41. 10.1377/hlthaff - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

