Randomized Phase II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium
- PMID: 34270348
- PMCID: PMC8547934
- DOI: 10.1200/JCO.21.00703
Randomized Phase II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium
Abstract
Purpose: 131I-metaiodobenzylguanidine (MIBG) is an active radiotherapeutic for neuroblastoma. The primary aim of this trial was to identify which of three MIBG regimens was likely associated with the highest true response rate.
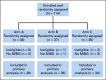
Patients and methods: Patients 1-30 years were eligible if they had relapsed or refractory neuroblastoma, at least one MIBG-avid site, and adequate autologous stem cells. Patients received MIBG 18 mCi/kg on day 1 and autologous stem cell on day 15. Patients randomly assigned to arm A received only MIBG; patients randomly assigned to arm B received intravenous vincristine on day 0 and irinotecan daily on days 0-4; patients randomly assigned to arm C received vorinostat (180 mg/m2/dose) orally once daily on days 1 to 12. The primary end point was response after one course by New Approaches to Neuroblastoma Therapy criteria. The trial was designed with 105 patients to ensure an 80% chance that the arm with highest response rate was selected.
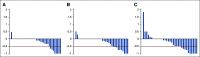
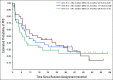
Results: One hundred fourteen patients were enrolled, with three ineligible and six unevaluable, leaving 105 eligible and evaluable patients (36 in arm A, 35 in arm B, and 34 in arm C; 55 boys; and median age 6.5 years). After one course, the response rates (partial response or better) on arms A, B, and C were 14% (95% CI, 5 to 30), 14% (5 to 31), and 32% (18 to 51). An additional five, five, and four patients met New Approaches to Neuroblastoma Therapy Minor Response criteria on arms A, B, and C, respectively. On arms A, B, and C, rates of any grade 3+ nonhematologic toxicity after first course were 19%, 49%, and 35%.
Conclusion: Vorinostat and MIBG is likely the arm with the highest true response rate, with manageable toxicity. Vincristine and irinotecan do not appear to improve the response rate to MIBG and are associated with increased toxicity.
Trial registration: ClinicalTrials.gov NCT02035137.
Conflict of interest statement
Figures

References
-
- Glowniak JV, Kilty JE, Amara SG, et al. : Evaluation of metaiodobenzylguanidine uptake by the norepinephrine, dopamine and serotonin transporters. J Nucl Med 34:1140-1146, 1993 - PubMed
-
- Wilson JS, Gains JE, Moroz V, et al. : A systematic review of 131I-meta iodobenzylguanidine molecular radiotherapy for neuroblastoma. Eur J Cancer 50:801-815, 2014 - PubMed
-
- Kraal KC, Bleeker GM, van Eck-Smit BL, et al. : Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer 76:188-196, 2017 - PubMed
-
- McCluskey AG, Boyd M, Ross SC, et al. : [131I]meta-iodobenzylguanidine and topotecan combination treatment of tumors expressing the noradrenaline transporter. Clin Cancer Res 11:7929-7937, 2005 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

