The Posology of Dupilumab in Pediatric Patients With Atopic Dermatitis
- PMID: 34270797
- PMCID: PMC9290854
- DOI: 10.1002/cpt.2366
The Posology of Dupilumab in Pediatric Patients With Atopic Dermatitis
Abstract
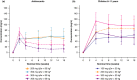
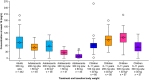
Dupilumab demonstrated efficacy with an acceptable safety profile in two randomized, double-blind, placebo-controlled, parallel-group, phase III trials in adolescents (12-17 years; LIBERTY AD ADOL) and children (6-11 years; LIBERTY AD PEDS) with atopic dermatitis (AD) treated for 16 weeks. Here, we present the pharmacokinetic profiles and exposure-response (E-R) relationships of dupilumab that guided the posology in these populations. A total of 251 adolescent patients with moderate-to-severe AD were randomized to subcutaneous dupilumab monotherapy every 2 weeks (q2w; 200 mg q2w, baseline weight < 60 kg; 300 mg q2w, ≥ 60 kg), dupilumab 300 mg every 4 weeks (q4w; non-weight tiered), or placebo; 367 children with severe AD were randomized to dupilumab q2w (100 mg q2w, baseline weight < 30 kg; 200 mg q2w, ≥ 30 kg), dupilumab 300 mg q4w, or placebo. Children received concomitant topical corticosteroids in addition to dupilumab, and loading doses were administered at the start of therapy. Mean dupilumab trough concentrations at week 16 for weight subcategories in each dosing regimen were compared with adult exposures for the approved dupilumab 300 mg q2w regimen. Positive E-R relationships were demonstrated between dupilumab trough concentrations and AD outcome measures across patient populations and regimens; no relationship was observed with treatment-emergent conjunctivitis. Based on these analyses, a weight-tiered posology was proposed for adolescents (200/300 mg q2w in patients 30-< 60 kg/≥ 60 kg) and children (300 mg q4w in patients 15-< 30 kg, 200 mg q2w in patients 30-< 60 kg) with moderate-to-severe AD.
Trial registration: ClinicalTrials.gov NCT03054428 NCT03345914.
© 2021 Regeneron Pharmaceuticals, Inc. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
M.A.K., P.K., M.P.K., K.S., Y.Z., M.R., C‐H.L., X.S., B.S., A.B., N.A‐H., and J.D.D. are employees and shareholders of Regeneron Pharmaceuticals, Inc. E.L.S. has been an advisor for Celgene and Merck; a consultant for Anacor Pharmaceuticals, Asubio, Celgene, Galderma, Genentech, Medicis Pharmaceutical, and Merck; and has received research support from Amgen, Celgene, Chugai, Galderma, and Regeneron Pharmaceuticals, Inc. A.S.P. has been a consultant for AbbVie, Abeona Therapeutics, Almirall, Asana BioSciences, Boehringer Ingelheim, BridgeBio, Dermavant, Dermira, Eli Lilly, Exicure, Forté, Incyte, InMed Pharmaceuticals, Janssen, LEO Pharma, LifeMax, Novartis, Pfizer, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc., Sanofi Genzyme, Sol‐Gel, and UCB; has been on the data safety monitoring board of Bausch, Galderma, and Novan; and a Principal Investigator (funding to institution) for AbbVie, AnaptysBio, Eli Lilly, Incyte, Janssen, Lenus Pharma, LEO Pharma, Novartis, Regeneron Pharmaceuticals, Inc, and UCB. E.C.S. has been a consultant for Dermavant, Eli Lilly, Pfizer, Regeneron Pharmaceuticals, Inc., and Verrica Pharmaceuticals; has been on the data safety monitoring board of GlaxoSmithKline, LEO Pharma, and Novan; and has been the Principal Investigator in clinical trials for Eli Lilly, Janssen, Regeneron Pharmaceuticals, Inc., Stiefel, and Verrica Pharmaceuticals. V.K. and C.X. are employees of and may hold stock options in Sanofi Genzyme.
Figures


References
-
- Odhiambo, J.A. , Williams, H.C. , Clayton, T.O. , Robertson, C.F. & Asher, M.I. & ISAAC Phase Three Study Group . Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J. Allergy Clin. Immunol. 124, 1251–1258 (2009). - PubMed
-
- Wollenberg, A. et al. ETFAD/EADV eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 30, 729–747 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

