Screening for Lung Cancer: CHEST Guideline and Expert Panel Report
- PMID: 34270968
- PMCID: PMC8727886
- DOI: 10.1016/j.chest.2021.06.063
Screening for Lung Cancer: CHEST Guideline and Expert Panel Report
Abstract
Background: Low-dose chest CT screening for lung cancer has become a standard of care in the United States, in large part because of the results of the National Lung Screening Trial (NLST). Additional evidence supporting the net benefit of low-dose chest CT screening for lung cancer, and increased experience in minimizing the potential harms, has accumulated since the prior iteration of these guidelines. Here, we update the evidence base for the benefit, harms, and implementation of low-dose chest CT screening. We use the updated evidence base to provide recommendations where the evidence allows, and statements based on experience and expert consensus where it does not.
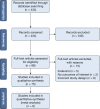
Methods: Approved panelists reviewed previously developed key questions using the Population, Intervention, Comparator, Outcome format to address the benefit and harms of low-dose CT screening, and key areas of program implementation. A systematic literature review was conducted using MEDLINE via PubMed, Embase, and the Cochrane Library on a quarterly basis since the time of the previous guideline publication. Reference lists from relevant retrievals were searched, and additional papers were added. Retrieved references were reviewed for relevance by two panel members. The quality of the evidence was assessed for each critical or important outcome of interest using the Grading of Recommendations, Assessment, Development, and Evaluation approach. Meta-analyses were performed when enough evidence was available. Important clinical questions were addressed based on the evidence developed from the systematic literature review. Graded recommendations and ungraded statements were drafted, voted on, and revised until consensus was reached.
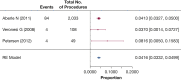
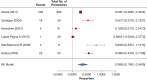
Results: The systematic literature review identified 75 additional studies that informed the response to the 12 key questions that were developed. Additional clinical questions were addressed resulting in seven graded recommendations and nine ungraded consensus statements.
Conclusions: Evidence suggests that low-dose CT screening for lung cancer can result in a favorable balance of benefit and harms. The selection of screen-eligible individuals, the quality of imaging and image interpretation, the management of screen-detected findings, and the effectiveness of smoking cessation interventions can impact this balance.
Keywords: guidelines; lung cancer; screening.
Copyright © 2021 American College of Chest Physicians. All rights reserved.
Figures
















References
-
- US Preventive Services Task Force Screening for lung cancer: US Preventative Services Task Force Recommendation Statement. JAMA. 2021;325(10):962–970. - PubMed
-
- Mazzone P.J., Silvestri G.A., Patel S., et al. Screening for lung cancer: CHEST Guideline and Expert Panel Report. Chest. 2018;153(4):954–985. - PubMed
-
- Gohagan J., Marcus P., Fagerstrom R., Pinsky P., Kramer B., Prorok P. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest. 2004;126(1):114–121. - PubMed

