Assessment of SARS-CoV-2 testing in children during a low prevalence period (VIGIL study 1)
- PMID: 34271254
- PMCID: PMC8276575
- DOI: 10.1016/j.idnow.2021.07.004
Assessment of SARS-CoV-2 testing in children during a low prevalence period (VIGIL study 1)
Abstract
Objectives: SARS-CoV-2 induces a broad spectrum of clinical manifestations, which overlap with other viral infections very common in children. We aimed to describe the percentage of positive SARS-CoV-2 RT-PCR tests in symptomatic and asymptomatic ambulatory children and to determine the predictive factors for positivity.
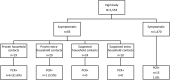
Patients and methods: From June 1 to July 31, 2020, we conducted a cross-sectional prospective, multicenter study (13 hospital emergency units and 59 ambulatory pediatricians) throughout France. Children under 15 years of age with a prescription of nasopharyngeal SARS-CoV-2 RT-PCR test were enrolled.
Results: Among the 1,553 RT-PCR tests, 22 were positive (1.4%; 95%CI [0.9; 2.1]). In both univariate and multivariate analyses, the predictive factors for positivity were age below 2 years (OR: 4.5 [1.6; 12.7]) and history of contact (OR: 12.3 [4.6; 32.8]).
Conclusions: In an epidemic stage with low SARS-CoV-2 circulation, sampling of children with nonspecific symptoms and without known contact could be questioned.
Keywords: Ambulatory; Children; Clinical signs; RT-PCR; SARS-CoV-2.
Copyright © 2021 Elsevier Masson SAS. All rights reserved.
Figures
References
-
- Levy et al C. Changes in reverse transcription polymerase chain reaction-positive severe acute respiratory syndrome coronavirus 2 rates in adults and children according to the epidemic stages. Pediatr Infect Dis J. 2020:e369–e372. doi: 10.1097/INF.0000000000002861. [Publish Ahead of Print] - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous