FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure
- PMID: 34272244
- PMCID: PMC8284898
- DOI: 10.1126/sciadv.abf4335
FOXC2 controls adult lymphatic endothelial specialization, function, and gut lymphatic barrier preventing multiorgan failure
Abstract
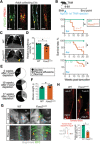
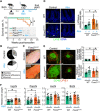
The mechanisms maintaining adult lymphatic vascular specialization throughout life and their role in coordinating inter-organ communication to sustain homeostasis remain elusive. We report that inactivation of the mechanosensitive transcription factor Foxc2 in adult lymphatic endothelium leads to a stepwise intestine-to-lung systemic failure. Foxc2 loss compromised the gut epithelial barrier, promoted dysbiosis and bacterial translocation to peripheral lymph nodes, and increased circulating levels of purine metabolites and angiopoietin-2. Commensal microbiota depletion dampened systemic pro-inflammatory cytokine levels, corrected intestinal lymphatic dysfunction, and improved survival. Foxc2 loss skewed the specialization of lymphatic endothelial subsets, leading to populations with mixed, pro-fibrotic identities and to emergence of lymph node-like endothelial cells. Our study uncovers a cross-talk between lymphatic vascular function and commensal microbiota, provides single-cell atlas of lymphatic endothelial subtypes, and reveals organ-specific and systemic effects of dysfunctional lymphatics. These effects potentially contribute to the pathogenesis of diseases, such as inflammatory bowel disease, cancer, or lymphedema.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures






References
-
- Petrova T. V., Koh G. Y., Biological functions of lymphatic vessels. Science 369, eaax4063 (2020). - PubMed
-
- González-Loyola A., Petrova T. V., Development and aging of the lymphatic vascular system. Adv. Drug Deliv. Rev. 169, 63–78 (2021). - PubMed
-
- Sabine A., Bovay E., Demir C. S., Kimura W., Jaquet M., Agalarov Y., Zangger N., Scallan J. P., Graber W., Gulpinar E., Kwak B. R., Makinen T., Martinez-Corral I., Ortega S., Delorenzi M., Kiefer F., Davis M. J., Djonov V., Miura N., Petrova T. V., FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J. Clin. Invest. 125, 3861–3877 (2015). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

