Individualized genetic makeup that controls natural killer cell function influences the efficacy of isatuximab immunotherapy in patients with multiple myeloma
- PMID: 34272304
- PMCID: PMC8287616
- DOI: 10.1136/jitc-2021-002958
Individualized genetic makeup that controls natural killer cell function influences the efficacy of isatuximab immunotherapy in patients with multiple myeloma
Abstract
Background: Phase IIb clinical trial with isatuximab (Isa)-lenalidomide (Len)-dexamethasone (Dex) showed an improved progression-free survival (PFS) in patients with relapsed or refractory multiple myeloma (RRMM), but the efficacy varied by patient. Antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) cells plays a crucial role in arbitrating antitumor activities of therapeutic-antibodies. We tested if patient-specific genetic makeup known to set NK cell functional threshold influence response to Isa-Len-Dex therapy.
Methods: We characterized 57 patients with RRMM receiving Isa-Len-Dex for polymorphisms of killer-cell immunoglobulin-like receptors (KIR), human leukocyte antigen (HLA) class I, and FCGR3A loci. In vitro ADCC assay, coincubating primary NK cells expressing specific KIR repertoire with multiple myeloma cell lines (MM cells) expressing selected HLA class I ligands, was used to confirm the identified genetic correlatives of clinical response.
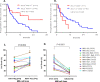
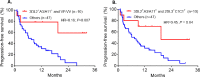
Results: Patients with KIR3DL2+ and its cognate-ligand HLA-A3/11+ had superior PFS than patients missing this combination (HR=0.43; p=0.02), while patients carrying KIR2DL1+ and HLA-C2C2+ compared with to patients missing this pair showed short PFS (HR=3.54; p=0.05). Patients with KIR3DL2+ and HLA-A3/11+ plus high-affinity FCGR3A-158V allele showed the most prolonged PFS (HR=0.35; p=0.007). Consistent with these clinical data, mechanistic experiments demonstrated that NK cells expressing KIR3DL2 trigger greater ADCC when MM cells express HLA-A3/11. Inversely, NK cells expressing KIR2DL1 do not kill if MM cells express the HLA-C2C2 ligand. NK cells expressing high-affinity FCGR3A-158VV-induced greater ADCC compared with those with low-affinity FCGR3A-158FF.
Conclusions: Our results suggest that KIR3DL2+ and HLA-A3/11+ with FCGR3A-158V markers lead to enhanced Isa-dependent NK-mediated cytolysis against MM cells and results in improved PFS in patients with RRMM treated by Isa-Len-Dex. Moreover, the presence of KIR2DL1+ and HLA-C2C2+ identifies patients who may have a lower response to Isa-Len-Dex therapy linked to a reduced NK-mediated ADCC. These biomarkers could potentially identify, via precision medicine, patients more likely to respond to Isa-Len-Dex immunotherapy.
Trial registration number: NCT01749969.
Keywords: genetic markers; immunity; immunotherapy; innate.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HS: no relationship to disclose. TGM: research funding: Sanofi. JM: no relationship to disclose. DK: no relationship to disclose. JK: no relationship to disclose. SM: employee of Sanofi. MC: employee of Sanofi. JLW: consulting or advisory role: Onyx/Amgen, Takeda, and Celgene. JMV: no relationship to disclose. RR: research funding: Sanofi; consulting or advisory role: CareDx.
Figures


References
-
- Michels TC, Petersen KE. Multiple myeloma: diagnosis and treatment. Am Fam Physician 2017;95:373–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
