Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration
- PMID: 34272449
- PMCID: PMC8285474
- DOI: 10.1038/s41598-021-94055-1
Multidisciplinary assessment of the Abbott BinaxNOW SARS-CoV-2 point-of-care antigen test in the context of emerging viral variants and self-administration
Abstract
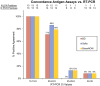
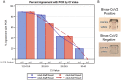
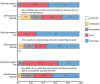
While there has been significant progress in the development of rapid COVID-19 diagnostics, as the pandemic unfolds, new challenges have emerged, including whether these technologies can reliably detect the more infectious variants of concern and be viably deployed in non-clinical settings as "self-tests". Multidisciplinary evaluation of the Abbott BinaxNOW COVID-19 Ag Card (BinaxNOW, a widely used rapid antigen test, included limit of detection, variant detection, test performance across different age-groups, and usability with self/caregiver-administration. While BinaxNOW detected the highly infectious variants, B.1.1.7 (Alpha) first identified in the UK, B.1.351 (Beta) first identified in South Africa, P.1 (Gamma) first identified in Brazil, B.1.617.2 (Delta) first identified in India and B.1.2, a non-VOC, test sensitivity decreased with decreasing viral loads. Moreover, BinaxNOW sensitivity trended lower when devices were performed by patients/caregivers themselves compared to trained clinical staff, despite universally high usability assessments following self/caregiver-administration among different age groups. Overall, these data indicate that while BinaxNOW accurately detects the new viral variants, as rapid COVID-19 tests enter the home, their already lower sensitivities compared to RT-PCR may decrease even more due to user error.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Ong DSY, de Man SJ, Lindeboom FA, Koeleman JGM. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin. Microbiol. Infect. 2020;26:1094 e1097–1094 e1010. doi: 10.1016/j.cmi.2020.05.028. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

