Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1-2 trial
- PMID: 34272481
- PMCID: PMC8727291
- DOI: 10.1038/s41375-021-01345-8
Long-term activity of tandem CD19/CD20 CAR therapy in refractory/relapsed B-cell lymphoma: a single-arm, phase 1-2 trial
Abstract
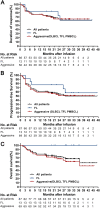
Increasing the remission rate and reducing the recurrence rate can improve the clinical efficacy of chimeric antigen receptor (CAR) T cell therapy in recurrent/refractory non-Hodgkin lymphoma (r/rNHL). In this open-label, single-arm phase I/II trial, 87 patients with r/rNHL, including 58 patients with aggressive diffuse large B-cell lymphoma and 24 with high tumour burden, received an infusion at doses of 0.5 × 106-8 × 106 TanCAR7 T cells per kilogram of body weight after conditioning chemotherapy. The best overall response rate was 78% (95% confidence interval [CI], 68-86); response rates were consistent across prognostic subgroups. The median follow-up was 27.7 months. The median progression-free survival was 27.6 months (95% CI, 11 to not reached). Cytokine release syndrome (CRS) occurred in 61 patients (70%) with 60% of cases being grade 1 or 2 and 10% being grade 3 or greater. Grade 3 CAR T cell-related encephalopathy syndrome (CRES) occurred in 2 patients (2%). Two patients died from treatment-associated severe pulmonary infection, and one died from CRS-related pulmonary injury between 1 and 3 months post infusion. Long-term remissions were observed following the use of TanCAR7 T cells in r/rNHL with a safety profile that included CRS but few cases of CRES.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32:3490–6. doi: 10.1200/JCO.2013.53.9593. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

