Deliberation, Dissent, and Distrust: Understanding Distinct Drivers of Coronavirus Disease 2019 Vaccine Hesitancy in the United States
- PMID: 34272559
- PMCID: PMC8406882
- DOI: 10.1093/cid/ciab633
Deliberation, Dissent, and Distrust: Understanding Distinct Drivers of Coronavirus Disease 2019 Vaccine Hesitancy in the United States
Abstract
Background: Despite the availability of safe and efficacious coronavirus disease 2019 vaccines, a significant proportion of the American public remains unvaccinated and does not appear to be immediately interested in receiving the vaccine.
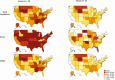
Methods: In this study, we analyzed data from the US Census Bureau's Household Pulse Survey, a biweekly cross-sectional survey of US households. We estimated the prevalence of vaccine hesitancy across states and nationally and assessed the predictors of vaccine hesitancy and vaccine rejection. In addition, we examined the underlying reasons for vaccine hesitancy, grouped into thematic categories.
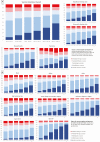
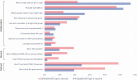
Results: A total of 459 235 participants were surveyed from 6 January to 29 March 2021. While vaccine uptake increased from 7.7% to 47%, vaccine hesitancy rates remained relatively fixed: overall, 10.2% reported that they would probably not get a vaccine and 8.2% that they would definitely not get a vaccine. Income, education, and state political leaning strongly predicted vaccine hesitancy. However, while both female sex and black race were factors predicting hesitancy, among those who were hesitant, these same characteristics predicted vaccine reluctance rather than rejection. Those who expressed reluctance invoked mostly "deliberative" reasons, while those who rejected the vaccine were also likely to invoke reasons of "dissent" or "distrust."
Conclusions: Vaccine hesitancy comprises a sizable proportion of the population and is large enough to threaten achieving herd immunity. Distinct subgroups of hesitancy have distinctive sociodemographic associations as well as cognitive and affective predilections. Segmented public health solutions are needed to target interventions and optimize vaccine uptake.
Keywords: COVID-19; COVID-19 vaccine; vaccine hesitancy.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

