Upcoming treatments for morphea
- PMID: 34272836
- PMCID: PMC8589364
- DOI: 10.1002/iid3.475
Upcoming treatments for morphea
Abstract
Morphea (localized scleroderma) is a rare autoimmune connective tissue disease with variable clinical presentations, with an annual incidence of 0.4-2.7 cases per 100,000. Morphea occurs most frequently in children aged 2-14 years, and the disease exhibits a female predominance. Insights into morphea pathogenesis are often extrapolated from studies of systemic sclerosis due to their similar skin histopathologic features; however, clinically they are two distinct diseases as evidenced by different demographics, clinical features, disease course and prognosis. An interplay between genetic factors, epigenetic modifications, immune and vascular dysfunction, along with environmental hits are considered as the main contributors to morphea pathogenesis. In this review, we describe potential new therapies for morphea based on both preclinical evidence and ongoing clinical trials. We focus on different classes of therapeutics, including antifibrotic, anti-inflammatory, cellular and gene therapy, and antisenolytic approaches, and how these target different aspects of disease pathogenesis.
Keywords: clinical trial; localized scleroderma; morphea; treatment.
© 2021 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
Jillian M. Richmond is an inventor on patent application #62489191, “Diagnosis and Treatment of Vitiligo” which covers targeting IL‐15 and Trm for the treatment of vitiligo, and on patent application #15/851,651, “Anti‐human CXCR3 antibodies for the Treatment of Vitiligo” which covers targeting CXCR3 for the treatment of vitiligo.
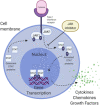
Figures

References
-
- Ferreli C, Gasparini G, Parodi A, Cozzani E, Rongioletti F, Atzori L. Cutaneous manifestations of scleroderma and scleroderma‐like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53(3):306‐336. - PubMed
-
- Weibel L, Sampaio MC, Visentin MT, Howell KJ, Woo P, Harper JI. Evaluation of methotrexate and corticosteroids for the treatment of localized scleroderma (morphoea) in children. Br J Dermatol. 2006;155(5):1013‐1020. - PubMed
-
- Zulian F, Vallongo C, Woo P, et al. Localized scleroderma in childhood is not just a skin disease. Arthritis Rheum. 2005;52(9):2873‐2881. - PubMed
-
- Christen‐Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385‐396. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

