Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial
- PMID: 34272849
- PMCID: PMC8530727
- DOI: 10.1210/clinem/dgab529
Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial
Abstract
Context: For children with growth hormone deficiency (GHD), treatment burden with daily somatropin injections [human growth hormone (hGH)] is high, which may lead to poor adherence and suboptimal overall treatment outcomes. Lonapegsomatropin (TransCon hGH) is an investigational long-acting, once-weekly prodrug for the treatment of GHD.
Objective: The objective of this study was to evaluate the efficacy and safety of once-weekly lonapegsomatropin vs daily somatropin.
Design: The heiGHt trial was a randomized, open-label, active-controlled, 52-week Phase 3 trial (NCT02781727).
Setting: This trial took place at 73 sites across 15 countries.
Patients: This trial enrolled and dosed 161 treatment-naïve, prepubertal patients with GHD.
Interventions: Patients were randomized 2:1 to receive lonapegsomatropin 0.24 mg hGH/kg/week or an equivalent weekly dose of somatropin delivered daily.
Main outcome measure: The primary end point was annualized height velocity (AHV) at week 52. Secondary efficacy end points included change from baseline in height SD scores (SDS).
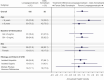
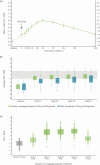
Results: Least squares (LS) mean (SE) AHV at 52 weeks was 11.2 (0.2) cm/year for lonapegsomatropin vs 10.3 (0.3) cm/year for daily somatropin (P = 0.009), with lonapegsomatropin demonstrating both noninferiority and superiority over daily somatropin. LS mean (SE) height SDS increased from baseline to week 52 by 1.10 (0.04) vs 0.96 (0.05) in the weekly lonapegsomatropin vs daily somatropin groups (P = 0.01). Bone age/chronological age ratio, adverse events, tolerability, and immunogenicity were similar between groups.
Conclusions: The trial met its primary objective of noninferiority in AHV and further showed superiority of lonapegsomatropin compared to daily somatropin, with similar safety, in treatment-naïve children with GHD.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



Comment in
-
Response to Letter to the Editor From L. Sävendahl et al: "Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial".J Clin Endocrinol Metab. 2022 Feb 17;107(3):e1333-e1334. doi: 10.1210/clinem/dgab738. J Clin Endocrinol Metab. 2022. PMID: 34626470 Free PMC article. No abstract available.
-
Letter to the Editor From Sävendahl et al.: "Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial".J Clin Endocrinol Metab. 2022 Jan 18;107(2):e888-e889. doi: 10.1210/clinem/dgab736. J Clin Endocrinol Metab. 2022. PMID: 34626473 Free PMC article. No abstract available.
-
Response to Letter to the Editor From Malozowski: "Weekly Lonapegsomatropin in Treatment-Naïve Children With Growth Hormone Deficiency: The Phase 3 heiGHt Trial".J Clin Endocrinol Metab. 2022 Apr 19;107(5):e2215-e2216. doi: 10.1210/clinem/dgab879. J Clin Endocrinol Metab. 2022. PMID: 34915563 Free PMC article. No abstract available.
-
Letter to the Editor From Malozowski: "Weekly Lonapegsomatropin in Treatment-naïve Children with Growth Hormone Deficiency: the Phase 3 heiGHt Trial".J Clin Endocrinol Metab. 2022 Apr 19;107(5):e2206-e2207. doi: 10.1210/clinem/dgab878. J Clin Endocrinol Metab. 2022. PMID: 34918085 Free PMC article. No abstract available.
References
-
- Deijen JB, van Driel MI, Drent ML. The involvement of the GH/IGF-I axis in cognitive functions of adult patients and healthy subjects. Open Endocrinol J. 2012;6:68-79.
-
- Hazem A, Elamin MB, Bancos I. Body composition and quality of life in adults treated with GH therapy: a systematic review and meta-analysis. Eur J Endocrinol. 2012;166(1):13-20. - PubMed
-
- Møller N, Jørgensen JO. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152-177. - PubMed
-
- Guyda HJ. Four decades of growth hormone therapy for short children: what have we achieved? J Clin Endocrinol Metab. 1999;84(12):4307-4316. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

