Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells
- PMID: 34273166
- PMCID: PMC9302892
- DOI: 10.1093/cvr/cvab244
Nicotine promotes vascular calcification via intracellular Ca2+-mediated, Nox5-induced oxidative stress, and extracellular vesicle release in vascular smooth muscle cells
Abstract
Aims: Smokers are at increased risk of cardiovascular events. However, the exact mechanisms through which smoking influences cardiovascular disease resulting in accelerated atherosclerosis and vascular calcification are unknown. The aim of this study was to investigate effects of nicotine on initiation of vascular smooth muscle cell (VSMC) calcification and to elucidate underlying mechanisms.
Methods and results: We assessed vascular calcification of 62 carotid lesions of both smoking and non-smoking patients using ex vivo micro-computed tomography (µCT) scanning. Calcification was present more often in carotid plaques of smokers (n = 22 of 30, 73.3%) compared to non-smokers (n = 11 of 32, 34.3%; P < 0.001), confirming higher atherosclerotic burden. The difference was particularly profound for microcalcifications, which was 17-fold higher in smokers compared to non-smokers. In vitro, nicotine-induced human primary VSMC calcification, and increased osteogenic gene expression (Runx2, Osx, BSP, and OPN) and extracellular vesicle (EV) secretion. The pro-calcifying effects of nicotine were mediated by Ca2+-dependent Nox5. SiRNA knock-down of Nox5 inhibited nicotine-induced EV release and calcification. Moreover, pre-treatment of hVSMCs with vitamin K2 ameliorated nicotine-induced intracellular oxidative stress, EV secretion, and calcification. Using nicotinic acetylcholine receptor (nAChR) blockers α-bungarotoxin and hexamethonium bromide, we found that the effects of nicotine on intracellular Ca2+ and oxidative stress were mediated by α7 and α3 nAChR. Finally, we showed that Nox5 expression was higher in carotid arteries of smokers and correlated with calcification levels in these vessels.
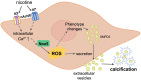
Conclusion: In this study, we provide evidence that nicotine induces Nox5-mediated pro-calcific processes as novel mechanism of increased atherosclerotic calcification. We identified that activation of α7 and α3 nAChR by nicotine increases intracellular Ca2+ and initiates calcification of hVSMCs through increased Nox5 activity, leading to oxidative stress-mediated EV release. Identifying the role of Nox5-induced oxidative stress opens novel avenues for diagnosis and treatment of smoking-induced cardiovascular disease.
Keywords: Nicotine Vascular calcification Vascular smooth muscle cell phenotypic switching Nox5 Vitamin K2.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures







References
-
- CDC / U.S. Department of health and human services. Surgeon General’s Report: The Health Consequences of Smoking—50 Years of Progress 2014;8:458-512.
-
- White WB. Smoking-related morbidity and mortality in the cardiovascular setting. Prev Cardiol 2007;10:1–4. - PubMed
-
- Willett WC, Green A, Stampfer MJ, Speizer FE, Colditz GA, Rosner B, Monson RR, Stason W, Hennekens CH.. Relative and absolute excess risks of coronary heart disease among women who smoke cigarettes. N Engl J Med 1987;317:1303–1309. - PubMed
-
- McEvoy JW, Blaha MJ, Defilippis AP, Lima JAC, Bluemke DA, Gregory Hundley W, Min JK, Shaw LJ, Lloyd-Jones DM, Graham Barr R, Budoff MJ, Blumenthal RS, Nasir K.. Cigarette smoking and cardiovascular events: role of inflammation and subclinical atherosclerosis from the multiethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 2015;35:700–709. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

