Cardiac pathology in COVID-19: a single center autopsy experience
- PMID: 34273507
- PMCID: PMC8278836
- DOI: 10.1016/j.carpath.2021.107370
Cardiac pathology in COVID-19: a single center autopsy experience
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is commonly associated with myocardial injury and heart failure. The pathophysiology behind this phenomenon remains unclear, with many diverse and multifaceted hypotheses. To contribute to this understanding, we describe the underlying cardiac findings in fifty patients who died with coronavirus disease 2019 (COVID-19).
Methods: Included were autopsies performed on patients with a positive SARS-CoV-2 reverse-transcriptase-polymerase-chain reaction test from the index hospitalization. In the case of out-of-hospital death, patients were included if post-mortem testing was positive. Complete autopsies were performed according to a COVID-19 safety protocol, and all patients underwent both macroscopic and microscopic examination. If available, laboratory findings and echocardiograms were reported.
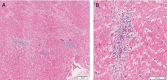
Results: The median age of the decedents was 63.5 years. The most common comorbidities included hypertension (90.0%), diabetes (56.0%) and obesity (50.0%). Lymphocytic inflammatory infiltrates in the heart were present in eight (16.0%) patients, with focal myocarditis present in two (4.0%) patients. Acute myocardial ischemia was observed in eight (16.0%) patients. The most common findings were myocardial fibrosis (80.0%), hypertrophy (72.0%), and microthrombi (66.0%). The most common causes of death were COVID-19 pneumonia in 18 (36.0%), COVID-19 pneumonia with bacterial superinfection in 12 (24.0%), and COVID-19 pneumonia with pulmonary embolism in 10 (20.0%) patients.
Conclusions: Cardiovascular comorbidities were prevalent, and pathologic changes associated with hypertensive and atherosclerotic cardiovascular disease were the most common findings. Despite markedly elevated inflammatory markers and cardiac enzymes, few patients exhibited inflammatory infiltrates or necrosis within cardiac myocytes. A unifying pathophysiologic mechanism behind myocardial injury in COVID-19 remains elusive, and additional autopsy studies are needed.
Keywords: Cardiac pathology; Cardiovascular disease; Coronavirus disease 2019; Severe acute respiratory syndrome coronavirus 2.
Copyright © 2021. Published by Elsevier Inc.
Figures




Similar articles
-
The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities.Cardiovasc Pathol. 2020 Sep-Oct;48:107233. doi: 10.1016/j.carpath.2020.107233. Epub 2020 May 7. Cardiovasc Pathol. 2020. PMID: 32434133 Free PMC article.
-
Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study.Circulation. 2021 Mar 9;143(10):1031-1042. doi: 10.1161/CIRCULATIONAHA.120.051828. Epub 2021 Jan 22. Circulation. 2021. PMID: 33480806
-
COVID-19 myocarditis: quantitative analysis of the inflammatory infiltrate and a proposed mechanism.Cardiovasc Pathol. 2021 Sep-Oct;54:107361. doi: 10.1016/j.carpath.2021.107361. Epub 2021 Jun 24. Cardiovasc Pathol. 2021. PMID: 34174415 Free PMC article.
-
Cardiac manifestations of COVID-19.Rev Cardiovasc Med. 2021 Jun 30;22(2):365-371. doi: 10.31083/j.rcm2202043. Rev Cardiovasc Med. 2021. PMID: 34258904 Review.
-
Cell-Specific Mechanisms in the Heart of COVID-19 Patients.Circ Res. 2023 May 12;132(10):1290-1301. doi: 10.1161/CIRCRESAHA.123.321876. Epub 2023 May 11. Circ Res. 2023. PMID: 37167361 Free PMC article. Review.
Cited by
-
Orthostatic Intolerance after COVID-19 Infection: Is Disturbed Microcirculation of the Vasa Vasorum of Capacitance Vessels the Primary Defect?Medicina (Kaunas). 2022 Dec 8;58(12):1807. doi: 10.3390/medicina58121807. Medicina (Kaunas). 2022. PMID: 36557009 Free PMC article.
-
MRI of cardiac involvement in COVID-19.Br J Radiol. 2024 Aug 1;97(1160):1367-1377. doi: 10.1093/bjr/tqae086. Br J Radiol. 2024. PMID: 38656976 Free PMC article. Review.
-
Profibrotic COVID-19 subphenotype exhibits enhanced localized ER-dependent HSP47+ expression in cardiac myofibroblasts in situ.J Mol Cell Cardiol. 2023 Dec;185:1-12. doi: 10.1016/j.yjmcc.2023.10.006. Epub 2023 Oct 14. J Mol Cell Cardiol. 2023. PMID: 37839656 Free PMC article.
-
Immune Cell-Based versus Albumin-Based Ratios as Outcome Predictors in Critically Ill COVID-19 Patients.J Inflamm Res. 2025 Jan 3;18:73-90. doi: 10.2147/JIR.S488972. eCollection 2025. J Inflamm Res. 2025. PMID: 39780984 Free PMC article.
-
COVID-19 and the Vasculature: Current Aspects and Long-Term Consequences.Front Cell Dev Biol. 2022 Feb 15;10:824851. doi: 10.3389/fcell.2022.824851. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35242762 Free PMC article. Review.
References
-
- Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648–1655. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

