Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO
- PMID: 34273539
- PMCID: PMC9257433
- DOI: 10.1016/j.freeradbiomed.2021.07.012
Human and rodent red blood cells do not demonstrate xanthine oxidase activity or XO-catalyzed nitrite reduction to NO
Abstract
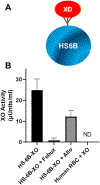
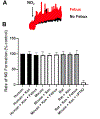
A number of molybdopterin enzymes, including xanthine oxidoreductase (XOR), aldehyde oxidase (AO), sulfite oxidase (SO), and mitochondrial amidoxime reducing component (mARC), have been identified as nitrate and nitrite reductases. Of these enzymes, XOR has been the most extensively studied and reported to be a substantive source of nitric oxide (NO) under inflammatory/hypoxic conditions that limit the catalytic activity of the canonical NOS pathway. It has also been postulated that XOR nitrite reductase activity extends to red blood cell (RBCs) membranes where it has been immunohistochemically identified. These findings, when combined with countervailing reports of XOR activity in RBCs, incentivized our current study to critically evaluate XOR protein abundance/enzymatic activity in/on RBCs from human, mouse, and rat sources. Using various protein concentrations of RBC homogenates for both human and rodent samples, neither XOR protein nor enzymatic activity (xanthine → uric acid) was detectable. In addition, potential loading of RBC-associated glycosaminoglycans (GAGs) by exposing RBC preparations to purified XO before washing did not solicit detectable enzymatic activity (xanthine → uric acid) or alter NO generation profiles. To ensure these observations extended to absence of XOR-mediated contributions to overall RBC-associated nitrite reduction, we examined the nitrite reductase activity of washed and lysed RBC preparations via enhanced chemiluminescence in the presence or absence of the XOR-specific inhibitor febuxostat (Uloric®). Neither addition of inhibitor nor the presence of the XOR substrate xanthine significantly altered the rates of nitrite reduction to NO by RBC preparations from either human or rodent sources confirming the absence of XO enzymatic activity. Furthermore, examination of the influence of the age (young cells vs. old cells) of human RBCs on XO activity also failed to demonstrate detectable XO protein. Combined, these data suggest: 1) that XO does not contribute to nitrite reduction in/on human and rodent erythrocytes, 2) care should be taken to validate immuno-detectable XO by demonstrating enzymatic activity, and 3) XO does not associate with human erythrocytic glycosaminoglycans or participate in nonspecific binding.
Keywords: Glycosaminoglycans; Nitric oxide; Nitrite; Red blood cells; Xanthine oxidoreductase.
Copyright © 2021. Published by Elsevier Inc.
Figures


References
-
- Nishino T, Okamoto K, The role of the [2Fe-2s] cluster centers in xanthine oxidoreductase, J. Inorg. Biochem 82 (2000) 43–49. - PubMed
-
- Iwasaki T, Okamoto K, Nishino T, Mizushima J, Hori H, Sequence motif-specific assignment of two [2Fe-2S] clusters in rat xanthine oxidoreductase studied by site-directed mutagenesis, J. Biochem 127 (2000) 771–778. - PubMed
-
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA, Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease, Proc. Natl. Acad. Sci. U. S. A 98 (2001) 15215–15220. - PMC - PubMed
-
- Farquharson CA, Butler R, Hill A, Belch JJ, Struthers AD, Allopurinol improves endothelial dysfunction in chronic heart failure, Circulation 106 (2002) 221–226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

