Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: a prospective placebo-controlled cross-over trial
- PMID: 34273987
- PMCID: PMC8286561
- DOI: 10.1186/s12974-021-02196-x
Intravenous administration of LPS activates the kynurenine pathway in healthy male human subjects: a prospective placebo-controlled cross-over trial
Abstract
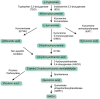
Background: Administration of lipopolysaccharide (LPS) from Gram-negative bacteria, also known as the human endotoxemia model, is a standardized and safe model of human inflammation. Experimental studies have revealed that peripheral administration of LPS leads to induction of the kynurenine pathway followed by depressive-like behavior and cognitive dysfunction in animals. The aim of the present study is to investigate how acute intravenous LPS administration affects the kynurenine pathway in healthy male human subjects.
Methods: The present study is a prospective, single-blinded, randomized, placebo-controlled cross-over study to investigate the effects of intravenously administered LPS (Escherichia coli O113, 2 ng/kg) on tryptophan and kynurenine metabolites over 48 h and their association with interleukin-6 (IL-6) and C-reactive protein (CRP). The study included 10 healthy, non-smoking men (18-40 years) free from medication. Statistical differences in tryptophan and kynurenine metabolites as well as associations with IL-6 and CRP in LPS and placebo treated subjects were assessed with linear mixed-effects models.
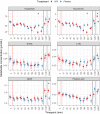
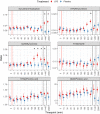
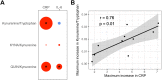
Results: Systemic injection of LPS was associated with significantly lower concentrations of plasma tryptophan and kynurenine after 4 h, as well as higher concentrations of quinolinic acid (QUIN) after 48 h compared to the placebo injection. No differences were found in kynurenic acid (KYNA) or picolinic acid plasma concentrations between LPS or placebo treatment. The KYNA/kynurenine ratio peaked at 6 h post LPS injection while QUIN/kynurenine maintained significantly higher from 3 h post LPS injection until 24 h. The kynurenine/tryptophan ratio was higher at 24 h and 48 h post LPS treatment. Finally, we report an association between the kynurenine/tryptophan ratio and CRP.
Conclusions: Our findings strongly support the concept that an inflammatory challenge with LPS induces the kynurenine pathway in humans, activating both the neurotoxic (QUIN) and neuroprotective (KYNA) branch of the kynurenine pathway.
Trial registration: This study is based on a study registered at ClinicalTrials.gov, NCT03392701 . Registered 21 December 2017.
Keywords: Experimental endotoxemia; Inflammation; Kynurenine metabolites; Lipopolysaccharides (LPS).
© 2021. The Author(s).
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

