Protocol for mapping psychosocial screening to resources in pediatric oncology: a pilot randomized controlled trial
- PMID: 34274016
- PMCID: PMC8285781
- DOI: 10.1186/s40814-021-00878-0
Protocol for mapping psychosocial screening to resources in pediatric oncology: a pilot randomized controlled trial
Abstract
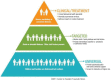
Background: A pediatric cancer diagnosis and its treatment can have a detrimental effect on the mental health of children and their families. Screening to identify psychosocial risk in families has been recognized as a standard of care in pediatric oncology, but there has been limited clinical application of this standard thus far. A significant impediment to the implementation of psychosocial screening is the dearth of information on how to translate psychosocial screening to clinical practice, and specifically, how to follow-up from screening results. This manuscript aims to describe a protocol of a new intervention examining the feasibility and acceptability of mapping via a Psychosocial Navigator (PSN) psychosocial screening results to specific recommendations of resources for families based on measured risk for psychosocial distress and mental health symptoms.
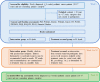
Methods: The pilot randomized control trial (RCT) consists of dyads of youth (10-17 years) newly diagnosed with cancer and their primary caregiver. This RCT includes two arms (intervention and control group), with each group completing measurements near diagnosis and 1 year later. After the initial assessment, dyads in the intervention group receive monthly screening results and recommendations from the study PSN that are tailored to these results. The patient's primary healthcare team (nurse, social worker, oncologist) also receive the risk, distress, and mental health results as well as the recommendations from the PSN.
Discussion: This study addresses a significant barrier to the implementation of psychosocial screening in pediatric oncology: specifically, the limited knowledge of how to follow-up from screening results. Findings from this pilot will inform a future definitive RCT to test the effectiveness of the intervention on patient and family mental health outcomes. This project has implications for enhancing clinical care in pediatric oncology, as well as other pediatric populations.
Strengths and limitations of this study: This is the first study of screening and follow-up using a psychosocial navigator. This study involves both patient and caregiver report. The small sample size necessitates a future larger study to investigate the effects of intervention.
Trial registration: NCT04132856 , Registered 10 October 2019-retrospectively registered.
Keywords: Mental health; Pediatric oncology; Protocol; Psychosocial; Screening.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Pilot randomized psychosocial trial of a screening intervention in pediatric oncology.Psychooncology. 2022 May;31(5):735-744. doi: 10.1002/pon.5857. Epub 2021 Nov 23. Psychooncology. 2022. PMID: 34813129 Clinical Trial.
-
A qualitative examination of the benefits and challenges of a psychosocial screening intervention in pediatric oncology: "Support comes to us".Pediatr Blood Cancer. 2022 May;69(5):e29578. doi: 10.1002/pbc.29578. Epub 2022 Jan 27. Pediatr Blood Cancer. 2022. PMID: 35084106
-
"Watch Me Grow- Electronic (WMG-E)" surveillance approach to identify and address child development, parental mental health, and psychosocial needs: study protocol.BMC Health Serv Res. 2021 Nov 17;21(1):1240. doi: 10.1186/s12913-021-07243-0. BMC Health Serv Res. 2021. PMID: 34789234 Free PMC article.
-
Implementation of family psychosocial risk assessment in pediatric cancer with the Psychosocial Assessment Tool (PAT): study protocol for a cluster-randomized comparative effectiveness trial.Implement Sci. 2020 Jul 29;15(1):60. doi: 10.1186/s13012-020-01023-w. Implement Sci. 2020. PMID: 32727493 Free PMC article.
-
Sharing psychosocial risk screening information with pediatric oncology healthcare providers: Service utilization and related factors.Pediatr Blood Cancer. 2022 Feb;69(2):e29456. doi: 10.1002/pbc.29456. Epub 2021 Dec 2. Pediatr Blood Cancer. 2022. PMID: 34854538 Clinical Trial.
Cited by
-
Beyond presence of symptoms: Self-reported psychosocial distress interference among outpatient youth with cancer and other life-limiting conditions.Pediatr Blood Cancer. 2024 Dec;71(12):e31273. doi: 10.1002/pbc.31273. Epub 2024 Sep 24. Pediatr Blood Cancer. 2024. PMID: 39317851 Free PMC article.
-
Protocol for evaluation of the feasibility and preliminary efficacy of a targeted transition readiness workshop intervention for pediatric brain tumor survivors.Pilot Feasibility Stud. 2024 Jan 19;10(1):11. doi: 10.1186/s40814-023-01437-5. Pilot Feasibility Stud. 2024. PMID: 38243344 Free PMC article.
-
Patient navigator programmes for children and adolescents with chronic diseases.Cochrane Database Syst Rev. 2024 Oct 9;10(10):CD014688. doi: 10.1002/14651858.CD014688.pub2. Cochrane Database Syst Rev. 2024. PMID: 39382077
-
Targeted Transition Readiness Workshops for Pediatric Brain Tumor Survivors: Feasibility, Acceptability, and Preliminary Effects.Curr Oncol. 2025 Jan 8;32(1):34. doi: 10.3390/curroncol32010034. Curr Oncol. 2025. PMID: 39851950 Free PMC article.
-
Mothers participation in caring for hospitalized children with acute gastroenteritis: A quasi-experimental study.Nurs Open. 2023 Sep;10(9):6398-6407. doi: 10.1002/nop2.1889. Epub 2023 Jun 15. Nurs Open. 2023. PMID: 37318191 Free PMC article.
References
-
- Kupst MJ, Patenaude AP. Coping and adaptation in pediatric cancer: Current perspectives. In: Abrams AN, Muriel AC, Wiener L, editors. Pediatric psychosocial oncology: Textbook for multidisciplinary care. Cham, Switzerland: Springer International Publishing; 2016. pp. 67–79.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials