Cellulosic biofuel production using emulsified simultaneous saccharification and fermentation (eSSF) with conventional and thermotolerant yeasts
- PMID: 34274018
- PMCID: PMC8285809
- DOI: 10.1186/s13068-021-02008-7
Cellulosic biofuel production using emulsified simultaneous saccharification and fermentation (eSSF) with conventional and thermotolerant yeasts
Abstract
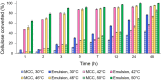
Background: Future expansion of corn-derived ethanol raises concerns of sustainability and competition with the food industry. Therefore, cellulosic biofuels derived from agricultural waste and dedicated energy crops are necessary. To date, slow and incomplete saccharification as well as high enzyme costs have hindered the economic viability of cellulosic biofuels, and while approaches like simultaneous saccharification and fermentation (SSF) and the use of thermotolerant microorganisms can enhance production, further improvements are needed. Cellulosic emulsions have been shown to enhance saccharification by increasing enzyme contact with cellulose fibers. In this study, we use these emulsions to develop an emulsified SSF (eSSF) process for rapid and efficient cellulosic biofuel production and make a direct three-way comparison of ethanol production between S. cerevisiae, O. polymorpha, and K. marxianus in glucose and cellulosic media at different temperatures.
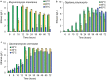
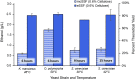
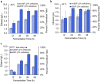
Results: In this work, we show that cellulosic emulsions hydrolyze rapidly at temperatures tolerable to yeast, reaching up to 40-fold higher conversion in the first hour compared to microcrystalline cellulose (MCC). To evaluate suitable conditions for the eSSF process, we explored the upper temperature limits for the thermotolerant yeasts Kluyveromyces marxianus and Ogataea polymorpha, as well as Saccharomyces cerevisiae, and observed robust fermentation at up to 46, 50, and 42 °C for each yeast, respectively. We show that the eSSF process reaches high ethanol titers in short processing times, and produces close to theoretical yields at temperatures as low as 30 °C. Finally, we demonstrate the transferability of the eSSF technology to other products by producing the advanced biofuel isobutanol in a light-controlled eSSF using optogenetic regulators, resulting in up to fourfold higher titers relative to MCC SSF.
Conclusions: The eSSF process addresses the main challenges of cellulosic biofuel production by increasing saccharification rate at temperatures tolerable to yeast. The rapid hydrolysis of these emulsions at low temperatures permits fermentation using non-thermotolerant yeasts, short processing times, low enzyme loads, and makes it possible to extend the process to chemicals other than ethanol, such as isobutanol. This transferability establishes the eSSF process as a platform for the sustainable production of biofuels and chemicals as a whole.
Keywords: Biofuels; Biomass pretreatment; Cellulose; Emulsions; Ethanol; Isobutanol; Kluyveromyces marxianus; Ogataea polymorpha; Optogenetics; SSF; Saccharomyces cerevisiae; Thermotolerant.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Selection of a thermotolerant Kluyveromyces marxianus strain with potential application for cellulosic ethanol production by simultaneous saccharification and fermentation.Appl Biochem Biotechnol. 2014 Feb;172(3):1553-64. doi: 10.1007/s12010-013-0612-5. Epub 2013 Nov 13. Appl Biochem Biotechnol. 2014. PMID: 24222495
-
Cellulosic ethanol production on temperature-shift simultaneous saccharification and fermentation using the thermostable yeast Kluyveromyces marxianus CHY1612.Bioprocess Biosyst Eng. 2012 Jan;35(1-2):115-22. doi: 10.1007/s00449-011-0621-0. Epub 2011 Sep 21. Bioprocess Biosyst Eng. 2012. PMID: 21947624
-
Selection of thermotolerant yeasts for simultaneous saccharification and fermentation (SSF) of cellulose to ethanol.Appl Biochem Biotechnol. 1991 Spring;28-29:307-15. doi: 10.1007/BF02922610. Appl Biochem Biotechnol. 1991. PMID: 1929369
-
Recent progress in engineering yeast producers of cellulosic ethanol.FEMS Yeast Res. 2025 Jan 30;25:foaf035. doi: 10.1093/femsyr/foaf035. FEMS Yeast Res. 2025. PMID: 40613832 Free PMC article. Review.
-
Cellulosic ethanol production: Progress, challenges and strategies for solutions.Biotechnol Adv. 2019 May-Jun;37(3):491-504. doi: 10.1016/j.biotechadv.2019.03.002. Epub 2019 Mar 5. Biotechnol Adv. 2019. PMID: 30849432 Review.
Cited by
-
Optimum blue light exposure: a means to increase cell-specific productivity in Chinese hamster ovary cells.Appl Microbiol Biotechnol. 2024 Dec 5;108(1):530. doi: 10.1007/s00253-024-13363-4. Appl Microbiol Biotechnol. 2024. PMID: 39636393 Free PMC article.
-
Positive selection of efficient ethanol producers from xylose at 45 °C in the yeast Ogataea polymorpha.Sci Rep. 2025 Jul 22;15(1):26530. doi: 10.1038/s41598-025-12204-2. Sci Rep. 2025. PMID: 40691245 Free PMC article.
-
Lipase Catalyzed Transesterification of Model Long-Chain Molecules in Double-Shell Cellulose-Coated Oil-in-Water Emulsion Particles as Microbioreactors.Int J Mol Sci. 2022 Oct 12;23(20):12122. doi: 10.3390/ijms232012122. Int J Mol Sci. 2022. PMID: 36292979 Free PMC article.
-
Cellulose-coated emulsion micro-particles self-assemble with yeasts for cellulose bio-conversion.Sci Rep. 2024 Mar 6;14(1):5499. doi: 10.1038/s41598-024-56204-0. Sci Rep. 2024. PMID: 38448579 Free PMC article.
References
-
- U.S. Energy Information Administration. How much ethanol is in gasoline, and how does it affect fuel economy? 2019.
-
- Langpap C, Wu JJ. Potential environmental impacts of increased reliance on corn-based bioenergy. Environ Resour Econ. 2011;49:147–171. doi: 10.1007/s10640-010-9428-8. - DOI
-
- Klemm D, Heublein B, Fink HP, Bohn A. Angew Chemie - Int. New York: Wiley; 2005. Cellulose: Fascinating biopolymer and sustainable raw material; pp. 3358–3393. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

