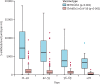
Spike-antibody waning after second dose of BNT162b2 or ChAdOx1
- PMID: 34274038
- PMCID: PMC8285117
- DOI: 10.1016/S0140-6736(21)01642-1
Spike-antibody waning after second dose of BNT162b2 or ChAdOx1
Conflict of interest statement
ACH serves on the UK New and Emerging Respiratory Virus Threats Advisory Group. All other authors declare no competing interests. The research costs for the study have been supported by the Medical Research Council Grant awarded to University College London. The study also received US$15 000 of Facebook advertising credit to support a pilot social media recruitment campaign on Aug 18, 2020. Virus Watch received funding via the UK Government Department of Health and Social Care's Vaccine Evaluation Programme to provide monthly Thriva antibody tests to adult participants. This work was supported by the Wellcome Trust through a Wellcome Clinical Research Career Development Fellowship to RWA. Author contributions and members of the Virus Watch Collaborative are listed in the appendix.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

