Inhibition of mitochondrial reactive oxygen species improves coronary endothelial function after cardioplegic hypoxia/reoxygenation
- PMID: 34274141
- PMCID: PMC8710187
- DOI: 10.1016/j.jtcvs.2021.06.029
Inhibition of mitochondrial reactive oxygen species improves coronary endothelial function after cardioplegic hypoxia/reoxygenation
Abstract
Objective: Cardioplegic ischemia-reperfusion and diabetes mellitus are correlated with coronary endothelial dysfunction and inactivation of small conductance calcium-activated potassium channels. Increased reactive oxidative species, such as mitochondrial reactive oxidative species, may contribute to oxidative injury. Thus, we hypothesized that inhibition of mitochondrial reactive oxidative species may protect coronary small conductance calcium-activated potassium channels and endothelial function against cardioplegic ischemia-reperfusion-induced injury.
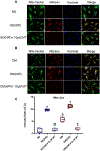
Methods: Small coronary arteries and endothelial cells from the hearts of mice with and without diabetes mellitus were isolated and examined by using a cardioplegic hypoxia and reoxygenation model to determine whether the mitochondria-targeted antioxidant Mito-Tempo could protect against coronary endothelial and small conductance calcium-activated potassium channel dysfunction. The microvessels or mouse heart endothelial cells were treated with or without Mito-Tempo (0-10 μM) 5 minutes before and during cardioplegic hypoxia and reoxygenation. Microvascular function was assessed in vitro by vessel myography. K+ currents of mouse heart endothelial cells were measured by whole-cell patch clamp. The levels of intracellular cytosolic free calcium (Ca2+) concentration, mitochondrial reactive oxidative species, and small conductance calcium-activated potassium protein expression of mouse heart endothelial cells were measured by Rhod-2 fluorescence staining, MitoSox, and Western blotting, respectively.
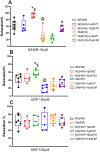
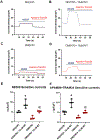
Results: Cardioplegic hypoxia and reoxygenation significantly attenuated endothelial small conductance calcium-activated potassium channel activity, caused calcium overload, and increased mitochondrial reactive oxidative species of mouse heart endothelial cells in both the nondiabetic and diabetes mellitus groups. In addition, treating mouse heart endothelial cells with Mito-Tempo (10 μM) reduced cardioplegic hypoxia and reoxygenation-induced Ca2+ and mitochondrial reactive oxidative species overload in both the nondiabetic and diabetes mellitus groups, respectively (P < .05). Treatment with Mito-Tempo (10 μM) significantly enhanced coronary relaxation responses to adenosine 5'-diphosphate and NS309 (P < .05), and endothelial small conductance calcium-activated potassium channel currents in both the nondiabetic and diabetes mellitus groups (P < .05).
Conclusions: Administration of Mito-Tempo improves endothelial function and small conductance calcium-activated potassium channel activity, which may contribute to its enhancement of endothelium-dependent vasorelaxation after cardioplegic hypoxia and reoxygenation.
Keywords: cardiac surgery; cardioplegia; cardioplegic arrest; cardioplegic hypoxia and reoxygenation; cardioplegic ischemia and reperfusion; cardiopulmonary bypass; coronary endothelial function; coronary endothelium-dependent vasodilation; coronary microcirculation; diabetes; endothelial function; ion channels; ischemia–reperfusion; mitochondria; mitochondrial reactive oxygen species; potassium channels; reactive oxygen species; small conductance calcium-activated potassium channels.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




Comment in
-
Commentary: Targeting mitochondrial injury after plegic arrest: SK-ipping the endothelial tempo or not?J Thorac Cardiovasc Surg. 2022 Nov;164(5):e228-e229. doi: 10.1016/j.jtcvs.2021.06.050. Epub 2021 Jun 29. J Thorac Cardiovasc Surg. 2022. PMID: 34274137 No abstract available.
-
Commentary: Protecting the powerhouse of the cell: The next frontier of myocardial protection?J Thorac Cardiovasc Surg. 2022 Nov;164(5):e227-e228. doi: 10.1016/j.jtcvs.2021.07.009. Epub 2021 Jul 14. J Thorac Cardiovasc Surg. 2022. PMID: 34303533 No abstract available.
References
-
- Tempe DK, Saigal D. Myocardial protection during cardiac surgery: An overview of cardioplegia: In: Sehgal R, Trikha A, eds. Yearbook of Anesthesiology-7. 2018;(Chapter 24):285–305.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

