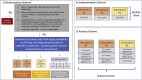
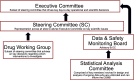
The COVID-19 Outpatient Pragmatic Platform Study (COPPS): Study design of a multi-center pragmatic platform trial
- PMID: 34274494
- PMCID: PMC8282451
- DOI: 10.1016/j.cct.2021.106509
The COVID-19 Outpatient Pragmatic Platform Study (COPPS): Study design of a multi-center pragmatic platform trial
Abstract
More than 3000 clinical trials related to COVID-19 have been registered through clinicaltrials.gov. With so many trials, there is a risk that many will be inconclusive due to being underpowered or due to an inability to recruit patients. At academic medical centers, multiple trials are competing for the same resources; the success of one may come at the expense of another. The COVID-19 Outpatient Pragmatic Protocol Study (COPPS) is a flexible phase 2, multi-site, randomized, blinded trial based at Stanford University designed to overcome these issues by simultaneously evaluating multiple COVID-19 treatments in the outpatient setting in one common platform with shared controls. This approach reduces the overall number of patients required for statistical power, while improving the likelihood that any enrolled patient receives active treatment. The platform study has two main domains designed to evaluate COVID-19 treatments by assessing their ability to reduce viral shedding (Viral Domain), measured with self-collected nasal swabs, or improve clinical outcomes (Clinical Domain), measured through self-reported symptomology data. Data are collected on both domains for all participants enrolled. Participants are followed over a 28-day period. COPPS has the advantage of pragmatism created around its workflow that is also appealing to potential participants because of a lower probability of inactive treatment. At the conclusion of this clinical trial we expect to have identified potentially effective therapeutic strategy/ies for treating COVID-19 in the outpatient setting, which will have a transformative impact on medicine and public health.
Keywords: Adaptive; COVID-19; Master; Phase 2; Platform; SARS-CoV-2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Eric Springman: Dr. Springman is a paid consultant for Celltaxis LLC.
Yvonne Maldonado: Stanford University School of Medicine has received funds from an anonymous donor for Dr. Maldonado to conduct a Phase 2 favipiravir study which should be completed by spring 2021.
No disclosure from other authors.
Figures
References
-
- Herper Matthew, Riglin Erin. Data shows panic and disorganization dominate the study of Covid-19 drugs. STAT. July 6, 2020 https://www.statnews.com/2020/07/06/data-show-panic-and-disorganization-... (accessed on September 20, 2020)
-
- Dunn A. Business Insider; 2020 Apr 24. There are Already 72 Drugs in Human Trials for Coronavirus in the US. With Hundreds More on the Way, A Top Drug Regulator Warns We Could Run Out of Researchers to Test Them All.https://www.businessinsider.com/fda-woodcock-overwhelming-amount-of-coro... (accessed on July 20, 2020)
-
- Herper Matthew. NIH to start “flurry” of large studies of potential Covid-19 treatment. STAT. July 23 2020 https://www.statnews.com/2020/07/23/nih-to-start-flurry-of-large-studies... (accessed on September 20, 2020)
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous