Adverse event burden in older patients with CLL receiving bendamustine plus rituximab or ibrutinib regimens: Alliance A041202
- PMID: 34274940
- PMCID: PMC8744070
- DOI: 10.1038/s41375-021-01342-x
Adverse event burden in older patients with CLL receiving bendamustine plus rituximab or ibrutinib regimens: Alliance A041202
Abstract
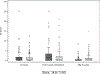
Ibrutinib has superior progression-free survival compared with bendamustine plus rituximab (BR) in older CLL patients, however, differences in treatment duration, six monthly BR cycles versus continuous ibrutinib, complicate adverse event (AE) comparisons. We introduce the AE burden score (AEsc) to compare AEs, calculated for each patient by summing over products of reporting period length and grade for each all-cause grade 1-4 AE and dividing by the length of time over which AEs are assessed. A total of 176 patients received BR and 361 ibrutinib alone or with six cycles of rituximab. At 38 months median follow-up, 64% remained on ibrutinib. Median AEsc was higher with BR versus ibrutinib in the first six cycles (7.2 versus 4.9, p < 0.0001). Within ibrutinib arms, median AEsc decreased significantly to 3.7 after six cycles (p < 0.0001). 10% and 14% of BR and ibrutinib patients discontinued treatment for AEs. In ibrutinib arms, cumulative incidence of grade 3 or higher atrial fibrillation, hypertension, and infection (AEs of clinical interest) at 12 months was 4.5%, 17.5%, and 12.8%, respectively, and increased more slowly thereafter to 7.7%, 25.4%, and 20.5% at 36 months. Analytical tools including the AEsc and cumulative incidence of AEs can help to better characterize AE burden over time. ClinicalTrials.gov identifier: NCT01886872.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing Interests
All other authors declare no competing interests.
Figures




References
-
- Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M. Chronic lymphocytic leukemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011; 22(Supplement 6): vi50–vi54. - PubMed
-
- Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009; 114(16): 3382–3391. - PubMed
-
- Eichhorst B, Fink AM, Bahlo J, Busch R, Math D, Kovacs G et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): an international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016. July; 17(7):928–942. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233331/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U24 CA114725/CA/NCI NIH HHS/United States
- R01 CA192928/CA/NCI NIH HHS/United States
- R01 CA177292/CA/NCI NIH HHS/United States
- UG1 CA232760/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- R01 CA183444/CA/NCI NIH HHS/United States
- R35 CA198183 /CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

