BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (the BIO-FLARE study): protocol for a non-randomised longitudinal cohort study
- PMID: 34275488
- PMCID: PMC8286860
- DOI: 10.1186/s41927-021-00194-3
BIOlogical Factors that Limit sustAined Remission in rhEumatoid arthritis (the BIO-FLARE study): protocol for a non-randomised longitudinal cohort study
Abstract
Background: Our knowledge of immune-mediated inflammatory disease (IMID) aetiology and pathogenesis has improved greatly over recent years, however, very little is known of the factors that trigger disease relapses (flares), converting diseases from inactive to active states. Focussing on rheumatoid arthritis (RA), the challenge that we will address is why IMIDs remit and relapse. Extrapolating from pathogenetic factors involved in disease initiation, new episodes of inflammation could be triggered by recurrent systemic immune dysregulation or locally by factors within the joint, either of which could be endorsed by overarching epigenetic factors or changes in systemic or localised metabolism.
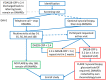
Methods: The BIO-FLARE study is a non-randomised longitudinal cohort study that aims to enrol 150 patients with RA in remission on a stable dose of non-biologic disease-modifying anti-rheumatic drugs (DMARDs), who consent to discontinue treatment. Participants stop their DMARDs at time 0 and are offered an optional ultrasound-guided synovial biopsy. They are studied intensively, with blood sampling and clinical evaluation at weeks 0, 2, 5, 8, 12 and 24. It is anticipated that 50% of participants will have a disease flare, whilst 50% remain in drug-free remission for the study duration (24 weeks). Flaring participants undergo an ultrasound-guided synovial biopsy before reinstatement of previous treatment. Blood samples will be used to investigate immune cell subsets, their activation status and their cytokine profile, autoantibody profiles and epigenetic profiles. Synovial biopsies will be examined to profile cell lineages and subtypes present at flare. Blood, urine and synovium will be examined to determine metabolic profiles. Taking into account all generated data, multivariate statistical techniques will be employed to develop a model to predict impending flare in RA, highlighting therapeutic pathways and informative biomarkers. Despite initial recruitment to time and target, the SARS-CoV-2 pandemic has impacted significantly, and a decision was taken to close recruitment at 118 participants with complete data.
Discussion: This study aims to investigate the pathogenesis of flare in rheumatoid arthritis, which is a significant knowledge gap in our understanding, addressing a major unmet patient need.
Trial registration: The study was retrospectively registered on 27/06/2019 in the ISRCTN registry 16371380 .
Keywords: DMARD cessation; DMARD withdrawal; Flare; Pathogenesis; Remission; Rheumatoid arthritis.
© 2021. The Author(s).
Conflict of interest statement
KFB, AGP, and JDI are named as inventors on a patent application filed by Newcastle University relating to the prediction of drug-free remission in rheumatoid arthritis (PCT/GB2019/050902). The remaining authors declare no competing interests.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

