Inadequate deltoid muscle penetration and concerns of improper COVID mRNA vaccine administration can be avoided by injection technique modification
- PMID: 34275671
- PMCID: PMC8249688
- DOI: 10.1016/j.vaccine.2021.06.081
Inadequate deltoid muscle penetration and concerns of improper COVID mRNA vaccine administration can be avoided by injection technique modification
Abstract
Background: Recent phase-3 clinical trials have demonstrated very encouraging results for mRNA based vaccines against COVID-19. Current FDA and manufacturer guidelines mandate intramuscular administration of these vaccines, as other administration routes may not provide the same levels of effectiveness and safety. Observing the vast amount of published media images of persons receiving their vaccines, the authors noted in many cases the injection technique involved skin bunching, raising concerns of inadequate deltoid muscle penetration and consequent lowered vaccine efficacy. Our study hypothesis was that skin bunching will increase the skin-to-muscle distance over 20 mm, the maximal distance allowing the required 5 mm muscle penetration with a 25 mm needle.
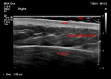
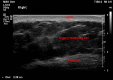
Materials and methods: 60 adult volunteers from our hospital staff were recruited, and using ultrasound, the skin-to-muscle distance measured in three positions: flat, skin bunching and muscle bunching. The skin-to-muscle distance difference and correlation with gender and BMI were calculated.
Results: Skin bunching significantly increased the skin-to-muscle distance in all subjects. In 6 (10%) subjects, this increase exceeded the 20 mm limit. Having a skin-to-deltoid distance of 20 mm or more strongly correlated with a BMI of 30 or more.
Conclusions: Skin bunching will prevent adequate intramuscular injection of vaccines in a small percentage of persons, but as hundreds of millions are expected to receive mRNA vaccines in the coming months, the multiplied result can have significant personal and societal consequences for millions of people globally, especially in obese populations, and therefore this practice should be strictly discouraged.
Keywords: COVID-19; Vaccine administration errors; mRNA vaccine.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- CDC. Pfizer-BioNTech COVID-19 Vaccine administration. https://wwwcdcgov/vaccines/covid-19/info-by-product/pfizer/indexhtml.
-
- CDC. Moderna COVID-19 Vaccine. https://wwwcdcgov/vaccines/covid-19/info-by-product/moderna/indexhtml.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

