Mechanistic Analysis of Age-Related Clinical Manifestations in Down Syndrome
- PMID: 34276349
- PMCID: PMC8281234
- DOI: 10.3389/fnagi.2021.700280
Mechanistic Analysis of Age-Related Clinical Manifestations in Down Syndrome
Abstract
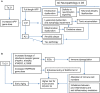
Down syndrome (DS) is the most common genetic cause of Alzheimer's disease (AD) due to trisomy for all or part of human chromosome 21 (Hsa21). It is also associated with other phenotypes including distinctive facial features, cardiac defects, growth delay, intellectual disability, immune system abnormalities, and hearing loss. All adults with DS demonstrate AD-like brain pathology, including amyloid plaques and neurofibrillary tangles, by age 40 and dementia typically by age 60. There is compelling evidence that increased APP gene dose is necessary for AD in DS, and the mechanism for this effect has begun to emerge, implicating the C-terminal APP fragment of 99 amino acid (β-CTF). The products of other triplicated genes on Hsa21 might act to modify the impact of APP triplication by altering the overall rate of biological aging. Another important age-related DS phenotype is hearing loss, and while its mechanism is unknown, we describe its characteristics here. Moreover, immune system abnormalities in DS, involving interferon pathway genes and aging, predispose to diverse infections and might modify the severity of COVID-19. All these considerations suggest human trisomy 21 impacts several diseases in an age-dependent manner. Thus, understanding the possible aging-related mechanisms associated with these clinical manifestations of DS will facilitate therapeutic interventions in mid-to-late adulthood, while at the same time shedding light on basic mechanisms of aging.
Keywords: Alzheimer’s disease; COVID-19; Down syndrome; hearing loss; infection; mechanisms.
Copyright © 2021 Chen, Xing, Chen, Salvi, Zhang, Tycko, Mobley and Yu.
Conflict of interest statement
WM is a patent holder of a potential treatment for Alzheimer disease held under his employer, the University of California, San Diego. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Aase J. M., Wilson A. C., Smith D. W. (1973). Small ears in Down’s syndrome: a helpful diagnostic aid. J. Pediatr. 82, 845–847. - PubMed
-
- Araya P., Waugh K. A., Sullivan K. D., Nunez N. G., Roselli E., Smith K. P., et al. . (2019). Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc. Natl. Acad. Sci. U S A 116, 24231–24241. 10.1073/pnas.1908129116 - DOI - PMC - PubMed

