Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective
- PMID: 34276410
- PMCID: PMC8278056
- DOI: 10.3389/fphys.2021.689747
Lactate Fluxes and Plasticity of Adipose Tissues: A Redox Perspective
Abstract
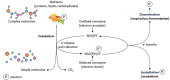
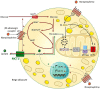
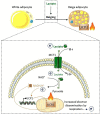
Lactate, a metabolite produced when the glycolytic flux exceeds mitochondrial oxidative capacities, is now viewed as a critical regulator of metabolism by acting as both a carbon and electron carrier and a signaling molecule between cells and tissues. In recent years, increasing evidence report its key role in white, beige, and brown adipose tissue biology, and highlights new mechanisms by which lactate participates in the maintenance of whole-body energy homeostasis. Lactate displays a wide range of biological effects in adipose cells not only through its binding to the membrane receptor but also through its transport and the subsequent effect on intracellular metabolism notably on redox balance. This study explores how lactate regulates adipocyte metabolism and plasticity by balancing intracellular redox state and by regulating specific signaling pathways. We also emphasized the contribution of adipose tissues to the regulation of systemic lactate metabolism, their roles in redox homeostasis, and related putative physiopathological repercussions associated with their decline in metabolic diseases and aging.
Keywords: adipose tissues; beige adipocytes; brown adipocytes; lactate; metabolic dialogs; redox metabolism; white adipocytes.
Copyright © 2021 Lagarde, Jeanson, Portais, Galinier, Ader, Casteilla and Carrière.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., et al. . (2010). The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253. 10.1152/ajpendo.00600.2009, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

