Habitat, Snow-Cover and Soil pH, Affect the Distribution and Diversity of Mortierellaceae Species and Their Associations to Bacteria
- PMID: 34276602
- PMCID: PMC8283828
- DOI: 10.3389/fmicb.2021.669784
Habitat, Snow-Cover and Soil pH, Affect the Distribution and Diversity of Mortierellaceae Species and Their Associations to Bacteria
Abstract
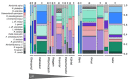
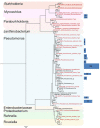
Mortierellaceae species are among the most frequent and globally distributed soil fungi. However, the factors shaping their diversity and distribution remain obscure. Several species have been reported to be associated to bacteria, but the kind and frequency of such associations were not addressed up to now. We hypothesized that such associations could be important for Mortierellaceae ecology. Therefore, our aim was to understand the driving factors responsible for the Mortierellaceae diversity, community composition and bacterial associations in alpine and subalpine habitats. For answering our question, we collected both snow-free and snow-covered soil at sampling sites from different habitats: bare alpine soil in a glacier forefield, alpine dwarf-willow habitats, and high-altitude Pinus cembra forests. The isolations were carried out by direct cultivation without any antibiotics to the isolation media. Altogether, we obtained 389 Mortierellaceae isolates representing 29 operational taxonomic units (OTUs). Many OTUs could be placed to the genera Mortierella sensu stricto, Dissophora, Entomortierella, Gamsiella, Linnemannia, and Podila, but others could not unambiguously be assigned to a genus. Our results demonstrate that both, the distribution as well as the diversity of the Mortierellaceae species, were significantly influenced by habitat, soil pH, and snow-cover. We noticed that >30% of our isolates were associated to a non-contaminant bacterium. The bacteria associated to our Mortierellaceae isolates belonged to seven different genera. Pseudomonas was the most frequently detected genus associated to the isolated Mortierellaceae species and it was found to be species-specific. Mortierellaceae-bacteria pairs, including those with Pseudomonas, were influenced by location, habitat, and snow-cover. The majority of the fungus-bacterium associations were potentially epihyphal, but we also detected potential endohyphal bacterial species belonging to Mycoavidus, Burkholderiaceae, and Paraburkholderia. Taken together, the non-random associations we detected suggest that fungus-bacterium associations are ecologically meaningful - an interesting path that needs to be investigated further.
Keywords: Mortierellomycotina; alpine; altitude; bacterial interactions; pH; psychrotolerant; sub-alpine; winter.
Copyright © 2021 Telagathoti, Probst and Peintner.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bergero R., Girlanda M., Varese G. C., Intili D., Luppi A. M. (1999). Psychrooligotrophic fungi from Arctic soils of Franz Joseph Land. Polar Biol. 21 361–368. 10.1007/s003000050374 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

