Discovery and Characterization of a Novel Bipartite Botrexvirus From the Phytopathogenic Fungus Botryosphaeria dothidea
- PMID: 34276630
- PMCID: PMC8280476
- DOI: 10.3389/fmicb.2021.696125
Discovery and Characterization of a Novel Bipartite Botrexvirus From the Phytopathogenic Fungus Botryosphaeria dothidea
Abstract
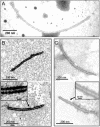
In this study, we describe a novel positive, single-stranded (+ss) RNA mycovirus, named Botryosphaeria dothidea botrexvirus 1 (BdBV1), from a phytopathogenic fungus Botryosphaeria dothidea showing abnormal morphology and attenuated virulence. BdBV1 is phylogenetically related to Botrytis virus X (BotVX) and is the second potential member of the proposed genus Botrexvirus in the family Alphaflexiviridae. However, it differs from the monopartite BotVX in that BdBV1 possesses a bipartite genome comprised of two ssRNA segments (RNA1 and RNA2 with lengths of 5,035 and 1,063 nt, respectively). BdBV1 RNA1 and RNA2 encode putative RNA-dependent RNA polymerase (RdRp) and coat protein (CP) genes, which share significant identity with corresponding genes in both fungal and plant viruses. Moreover, open reading frames (ORFs) 2-4 of BdBV1 RNA1 shared no detectable identity with any known viral proteins. Immunosorbent electron microscopy (ISEM) analysis using an antibody against the virus CP generated in vitro revealed that BdBV1 is encapsidated in filamentous particles. A comparison of the biological effects of BdBV1 infection on symptoms and growth in isogenic lines of virus-free and virus-infected B. dothidea revealed that BdBV1 is probably involved in reduced growth and virulence of the host fungus. This study describes and characterizes a novel bipartite botrexvirus, which is closely related to uni- and multi-partite fungal and plant viruses and contributes useful information to a better understanding of virus evolution.
Keywords: Alphaflexiviridae; Botryosphaeria dothidea; Botryosphaeria dothidea botrexvirus 1; bipartite botrexvirus; filamentous particles; genome.
Copyright © 2021 Yang, Xu, Zhou, Yang, Wang, Xiao, Guo, Hong and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

