Detection of Immune Checkpoint Receptors - A Current Challenge in Clinical Flow Cytometry
- PMID: 34276685
- PMCID: PMC8281132
- DOI: 10.3389/fimmu.2021.694055
Detection of Immune Checkpoint Receptors - A Current Challenge in Clinical Flow Cytometry
Abstract
Immunological therapy principles are increasingly determining modern medicine. They are used to treat diseases of the immune system, for tumors, but also for infections, neurological diseases, and many others. Most of these therapies base on antibodies, but small molecules, soluble receptors or cells and modified cells are also used. The development of immune checkpoint inhibitors is amazingly fast. T-cell directed antibody therapies against PD-1 or CTLA-4 are already firmly established in the clinic. Further targets are constantly being added and it is becoming increasingly clear that their expression is not only relevant on T cells. Furthermore, we do not yet have any experience with the long-term systemic effects of the treatment. Flow cytometry can be used for diagnosis, monitoring, and detection of side effects. In this review, we focus on checkpoint molecules as target molecules and functional markers of cells of the innate and acquired immune system. However, for most of the interesting and potentially relevant parameters, there are still no test kits suitable for routine use. Here we give an overview of the detection of checkpoint molecules on immune cells in the peripheral blood and show examples of a possible design of antibody panels.
Keywords: autoimmunity; checkpoint receptors; flow cytometry; immune diagnostics; immune oncology; immunity; infection; laboratory diagnose.
Copyright © 2021 Shibru, Fey, Fricke, Blaudszun, Fürst, Weise, Seiffert, Weyh, Köhl, Sack and Boldt.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

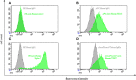
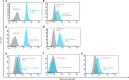
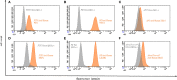

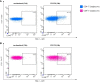
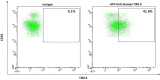
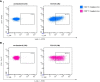
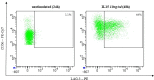

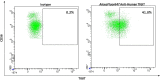

References
-
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/Eu (2017).
-
- Lambert C, Yanikkaya Demirel G, Keller T, Preijers F, Psarra K, Schiemann M, et al. . Flow Cytometric Analyses of Lymphocyte Markers in Immune Oncology: A Comprehensive Guidance for Validation Practice According to Laws and Standards. Front Immunol (2020) 11:2169. 10.3389/fimmu.2020.02169 - DOI - PMC - PubMed
-
- Özcürümez MK, Haeckel R, Gurr E, Streichert T, Sack U. Determination and Verification of Reference Interval Limits in Clinical Chemistry. Recommendations for Laboratories on Behalf of the Working Group Guide Limits of the DGKL With Respect to ISO Standard 15189 and the Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations (Rili-Baek). J Lab Med (2019) 43:127–33. 10.1515/labmed-2018-0500 - DOI
-
- ISO . Iso 15189-2012 Medical Laboratories - Requirements for Quality and Competence. Geneva: ISO; (2012).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

