Expression Profiling and Functional Analysis of Candidate Col10a1 Regulators Identified by the TRAP Program
- PMID: 34276786
- PMCID: PMC8283764
- DOI: 10.3389/fgene.2021.683939
Expression Profiling and Functional Analysis of Candidate Col10a1 Regulators Identified by the TRAP Program
Abstract
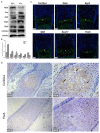
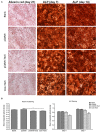
Hypertrophic chondrocytes and their specific marker, the type X collagen gene (Col10a1), are critical components of endochondral bone formation during skeletal development. We previously found that Runx2 is an indispensable mouse Col10a1 gene regulator and identified many other transcription factors (TFs) that potentially interact with the 150-bp Col10a1 cis-enhancer. However, the roles of these candidate TFs in Col10a1 expression and chondrocyte hypertrophy have not been elucidated. Here, we focus on 32 candidate TFs recently identified by analyzing the 150-bp Col10a1 enhancer using the transcription factor affinity prediction (TRAP) program. We found that 12 TFs (Hoxa3, Lsx, Evx2, Dlx5, S8, Pax2, Egr2, Mef2a, Barhl2, GKlf, Sox17, and Crx) were significantly upregulated and four TFs (Lhx4, Tbx5, Mef2c, and Hb9) were significantly downregulated in hypertrophic MCT cells, which show upregulation of Col10a1 expression. Most of the differential expression pattern of these TFs conformed with the results obtained from ATDC5 cell model and primary mouse chondrocytes. Notably, Tbx5 was downregulated upon Col10a1 upregulation, overexpression of Tbx5 decreased Col10a1 expression, and knock-down of Tbx5 increased Col10a1 expression in hypertrophic chondrocytes, suggesting that Tbx5 is a negative regulator of Col10a1. We further generated a stable Tbx5-overexpressing ATDC5 cell line and ColX-Tbx5 transgenic mice driven by Col10a1-specific enhancers and promoters. Tbx5 overexpression decreased Col10a1 expression in ATDC5 cells cultured as early as day 7 and in limb tissue on post-natal day 1. Slightly weaker alkaline phosphatase staining was also observed in cell culture on day 7 and in limb digits on embryonic day 17.5, indicating mildly delayed ossification. Further characterization of these candidate Col10a1 transcriptional regulators could help identify novel therapeutic targets for skeletal diseases associated with abnormal chondrocyte hypertrophy.
Keywords: Col10a1 regulators; Runx2; TRAP program; Tbx-5; chondrocyte hypertrophy; skeletal disease.
Copyright © 2021 Bian, Zhu, Liang, Hei, Zhang, Li, Chen, Lu, Gu, Qiao and Zheng.
Conflict of interest statement
YLu and QZ were employed by company Shenzhen Academy of Peptide Targeting Technology at Pingshan and Shenzhen Tyercan Bio-pharm Co., Ltd., Shenzhen, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

