Bronchopulmonary Dysplasia Predicted by Developing a Machine Learning Model of Genetic and Clinical Information
- PMID: 34276789
- PMCID: PMC8283015
- DOI: 10.3389/fgene.2021.689071
Bronchopulmonary Dysplasia Predicted by Developing a Machine Learning Model of Genetic and Clinical Information
Abstract
Background: An early and accurate evaluation of the risk of bronchopulmonary dysplasia (BPD) in premature infants is pivotal in implementing preventive strategies. The risk prediction models nowadays for BPD risk that included only clinical factors but without genetic factors are either too complex without practicability or provide poor-to-moderate discrimination. We aim to identify the role of genetic factors in BPD risk prediction early and accurately.
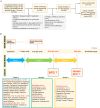
Methods: Exome sequencing was performed in a cohort of 245 premature infants (gestational age <32 weeks), with 131 BPD infants and 114 infants without BPD as controls. A gene burden test was performed to find risk genes with loss-of-function mutations or missense mutations over-represented in BPD and severe BPD (sBPD) patients, with risk gene sets (RGS) defined as BPD-RGS and sBPD-RGS, respectively. We then developed two predictive models for the risk of BPD and sBPD by integrating patient clinical and genetic features. The performance of the models was evaluated using the area under the receiver operating characteristic curve (AUROC).
Results: Thirty and 21 genes were included in BPD-RGS and sBPD-RGS, respectively. The predictive model for BPD, which combined the BPD-RGS and basic clinical risk factors, showed better discrimination than the model that was only based on basic clinical features (AUROC, 0.915 vs. AUROC, 0.814, P = 0.013, respectively) in the independent testing dataset. The same was observed in the predictive model for sBPD (AUROC, 0.907 vs. AUROC, 0.826; P = 0.016).
Conclusion: This study suggests that genetic information contributes to susceptibility to BPD. The predictive model in this study, which combined BPD-RGS with basic clinical risk factors, can thus accurately stratify BPD risk in premature infants.
Keywords: bronchopulmonary dysplasia; exome sequencing; machine learning; prediction model; premature infants.
Copyright © 2021 Dai, Chen, Dong, Chen, Mei, Lu, Yang, Wu, Cao, Wang, Zhou and Qian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Carrera P., Di Resta C., Volonteri C., Castiglioni E., Bonfiglio S., Lazarevic D., et al. (2015). BPD and genetics study group. exome sequencing and pathway analysis for identification of genetic variability relevant for bronchopulmonary dysplasia (BPD) in preterm newborns: a pilot study. Clin. Chim. Acta 451(Pt A) 39–45. 10.1016/j.cca.2015.01.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources

