EPR of Photoexcited Triplet-State Acceptor Porphyrins
- PMID: 34276860
- PMCID: PMC8279703
- DOI: 10.1021/acs.jpcc.1c03278
EPR of Photoexcited Triplet-State Acceptor Porphyrins
Abstract
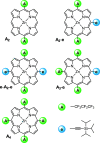
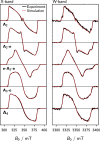
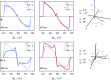
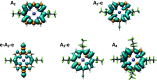
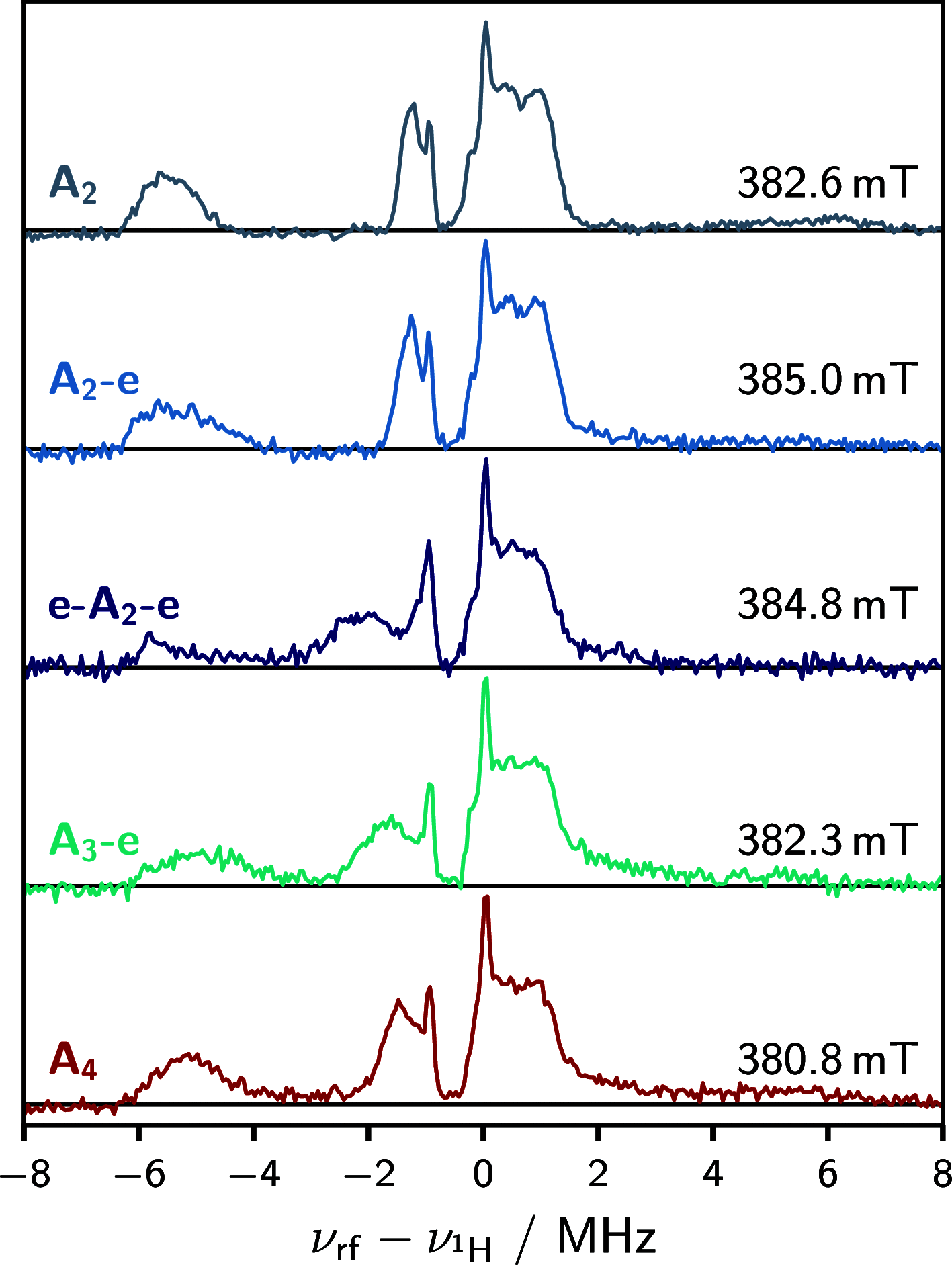
The photoexcited triplet states of porphyrin architectures are of significant interest in a wide range of fields including molecular wires, nonlinear optics, and molecular spintronics. Electron paramagnetic resonance (EPR) is a key spectroscopic tool in the characterization of these transient paramagnetic states singularly well suited to quantify spin delocalization. Previous work proposed a means of extracting the absolute signs of the zero-field splitting (ZFS) parameters, D and E, and triplet sublevel populations by transient continuous wave, hyperfine measurements, and magnetophotoselection. Here, we present challenges of this methodology for a series of meso-perfluoroalkyl-substituted zinc porphyrin monomers with orthorhombic symmetries, where interpretation of experimental data must proceed with caution and the validity of the assumptions used in the analysis must be scrutinized. The EPR data are discussed alongside quantum chemical calculations, employing both DFT and CASSCF methodologies. Despite some success of the latter in quantifying the magnitude of the ZFS interaction, the results clearly provide motivation to develop improved methods for ZFS calculations of highly delocalized organic triplet states.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Anderson H. L. Building Molecular Wires from the Colours of Life: Conjugated Porphyrin Oligomers. Chem. Commun. 1999, 2323–2330. 10.1039/a904209a. - DOI
-
- Bakulin A. A.; Rao A.; Pavelyev V. G.; van Loosdrecht P. H.; Pshenichnikov M. S.; Niedzialek D.; Cornil J.; Beljonne D.; Friend R. H. The Role of Driving Energy and Delocalized States for Charge Separation in Organic Semiconductors. Science 2012, 335, 1340–1344. 10.1126/science.1217745. - DOI - PubMed
LinkOut - more resources
Full Text Sources
