Efficient synthesis of antiviral agent uprifosbuvir enabled by new synthetic methods
- PMID: 34276931
- PMCID: PMC8261776
- DOI: 10.1039/d1sc01978c
Efficient synthesis of antiviral agent uprifosbuvir enabled by new synthetic methods
Abstract
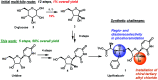
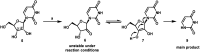
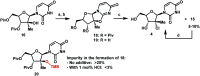
An efficient route to the HCV antiviral agent uprifosbuvir was developed in 5 steps from readily available uridine in 50% overall yield. This concise synthesis was achieved by development of several synthetic methods: (1) complexation-driven selective acyl migration/oxidation; (2) BSA-mediated cyclization to anhydrouridine; (3) hydrochlorination using FeCl3/TMDSO; (4) dynamic stereoselective phosphoramidation using a chiral nucleophilic catalyst. The new route improves the yield of uprifosbuvir 50-fold over the previous manufacturing process and expands the tool set available for synthesis of antiviral nucleotides.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
-
- HCV: The Journey from Discovery to a Cure, ed. M. J. Sofia, Top. Med. Chem., 2019, vol. 32
-
- Alexandre F.-R. Badaroux E. Bilello J. P. Bot S. Bouisset T. Brandt G. Cappelle S. Chapron C. Chaves D. Convard T. Counor C. Da Costa D. Dukhan D. Gay M. Gosselin G. Griffon J.-F. Gupta K. Hernandez-Santiago B. La Colla M. Lioure M.-P. Milhau J. Paparin J.-L. Peyronnet J. Parsy C. Rouvière C. P. Rahali H. Rahali R. Salanson A. Seifer M. Serra I. Standring D. Surleraux D. Dousson C. B. Bioorg. Med. Chem. Lett. 2017;27:4323–4330. - PubMed
LinkOut - more resources
Full Text Sources

