Rapid and robust cysteine bioconjugation with vinylheteroarenes
- PMID: 34276935
- PMCID: PMC8261766
- DOI: 10.1039/d1sc02722k
Rapid and robust cysteine bioconjugation with vinylheteroarenes
Abstract
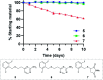
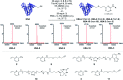
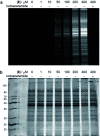
Methods for residue-selective and stable modification of canonical amino acids enable the installation of distinct functionality which can aid in the interrogation of biological processes or the generation of new therapeutic modalities. Herein, we report an extensive investigation of reactivity and stability profiles for a series of vinylheteroarene motifs. Studies on small molecule and protein substrates identified an optimum vinylheteroarene scaffold for selective cysteine modification. Utilisation of this lead linker to modify a number of protein substrates with various functionalities, including the synthesis of a homogeneous, stable and biologically active antibody-drug conjugate (ADC) was then achieved. The reagent was also efficient in labelling proteome-wide cysteines in cell lysates. The efficiency and selectivity of these reagents as well as the stability of the products makes them suitable for the generation of biotherapeutics or studies in chemical biology.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

