A molecular twist on hydrophobicity
- PMID: 34276953
- PMCID: PMC8261874
- DOI: 10.1039/d1sc02673a
A molecular twist on hydrophobicity
Abstract
A thorough exploration of the molecular basis for hydrophobicity with a comprehensive set of theoretical tools and an extensive set of organic solvent S/water binary systems is discussed in this work. Without a single exception, regardless of the nature or structure of S, all quantum descriptors of bonding yield stabilizing S⋯water interactions, therefore, there is no evidence of repulsion and thus no reason for etymological hydrophobicity at the molecular level. Our results provide molecular insight behind the exclusion of S molecules by water, customarily invoked to explain phase separation and the formation of interfaces, in favor of a complex interplay between entropic, enthalpic, and dynamic factors. S⋯water interfaces are not just thin films separating the two phases; instead, they are non-isotropic regions with density gradients for each component whose macroscopic stability is provided by a large number of very weak dihydrogen contacts. We offer a definition of interface as the region in which the density of the components in the A/B binary system is not constant. At a fundamental level, our results contribute to better current understanding of hydrophobicity.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



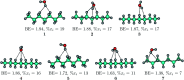
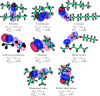
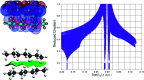
References
-
- Compendium of Chemical Terminology, accessed: 2020-05-12, DOI: 10.1351/goldbook
-
- Budavari S., O'Neil M., Smith A., Heckelman P. and Kinneary J., The Merck Index, An encyclopedia of chemicals, drugs and biologicals, Merck Research Laboratories Division of Merck & Co; Inc. Monograph, Whitehouse Station, NJ, 1996, vol. 4175
-
- Lanza G. Chiacchio M. A. The water molecule arrangement over the side chain of some aliphatic amino acids: A quantum chemical and bottom-up investigation. Int. J. Quantum Chem. 2020;120:e26161.
-
- Duarte Alaniz V. Rocha-Rinza T. Cuevas G. Assessment of hydrophobic interactions and their contributions through the analysis of the methane dimer. J. Comput. Chem. 2015;36:361–375. - PubMed
-
- Rojas-Valencia N. Gómez S. Montillo S. Manrique-Moreno M. Cappelli C. Hadad C. Restrepo A. Evolution of Bonding during the Insertion of Anionic Ibuprofen into Model Cell Membranes. J. Phys. Chem. B. 2020;124:79–90. - PubMed
LinkOut - more resources
Full Text Sources

