The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions From Routine Practice
- PMID: 34277420
- PMCID: PMC8283700
- DOI: 10.3389/fonc.2021.675296
The WHO 2018 Classification of Cutaneous Melanocytic Neoplasms: Suggestions From Routine Practice
Abstract
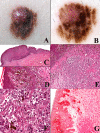
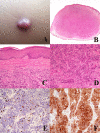
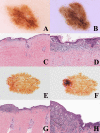
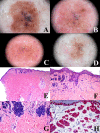
The "multidimensional" World Health Organization (WHO) classification 2018 of melanocytic tumors encompasses nine melanoma pathways (seven of which for cutaneous melanoma) according to a progression model in which morphologically intermediate melanocytic tumors are cosidered as simulators and/or precursors to melanoma. These "intermediates" can be subclassified into: i) a "classical" subgroup (superficial/thin compound: dysplastic nevus), which is placed within the morphologic and molecular progression spectrum of classical (Clark's and McGovern's) melanoma subtypes (superficial spreading and, possibly, nodular); and ii) a "non-classical" subgroup (thick compound/dermal: "melanocytomas") whose genetic pathways diverge from classical melanoma subtypes. Such a progression model is aimed at giving a conceptual framework for a histopathological classification; however, routine clinicopathological practice strongly suggests that most melanomas arise de novo and that the vast majority of nevi are clinically stable or even involuting over time. Clinicopathological correlation can help identify some severely atypical but benign tumors (e.g.: sclerosing nevus with pseudomelanomatous features) as well as some deceptively bland melanomas (e.g.: lentiginous melanoma; nested melanoma), thereby addressing some ambiguous cases to a correct clinical management. The recently available adjuvant therapy regimens for melanoma raise the problem of a careful distinction between severely atypical (high grade) melanocytoma and "classical" melanoma: conventional morphology can guide an algorithmic approach based on an antibody panel (anti-mutated BRAF, BAP1, PRAME, ALK, TRKA, MET, HRAS-WT, ROS; beta catenin; R1alpha; p16; HMB45; Ki67), a first-line molecular study (identification of hot spot mutations of BRAF and NRAS) and an advanced molecular study (sequencing of NF1, KIT, BRAF, MAP2K1, GNAQ, GNA11, PLCB4, CYSLTR2, HRAS; fusions studies of BRAF, RET, MAP3K8, PRKCA); as a final step, next-generation sequencing can identify melanocytic tumors with rare genetic signatures and melanocytic tumors with a high tumor mutation burden which should be definitely ascribed to the category of classical melanoma with the respective therapeutic options.
Keywords: clinicopathological correlation; dysplastic nevus; histopathology; immunohistochemistry; melanocytoma; melanoma; molecular biology.
Copyright © 2021 Ferrara and Argenziano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Elder DE, Massi D, Scolyer RA, Willemze R. Who Classification of Skin Tumours, 4th Edition. Lyon: IARC; (2018).
-
- Ferrara G. “The Histopathological Gray Zone”. In: Argenziano G, Lallas A, Longo C, Moscarella E, Kyrgidis A, Ferrara G, editors. Cutaneous Melanoma: A Pocket Guide for Diagnosis and Management. London, UK: Academic Press; (2017). p. 155–89.
-
- Cerroni L, Barnhill R, Elder D, Gottlieb G, Heenan P, Kutzner H, et al. . Melanocytic Tumors of Uncertain Malignant Potential: Results of a Tutorial Held at the XXIX Symposium of the International Society of Dermatopathology in Graz, October 2008. Am J Surg Pathol (2010) 34:314–26. 10.1097/PAS.0b013e3181cf7fa0 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

