Management and Clinical Outcome of Posterior Reversible Encephalopathy Syndrome in Pediatric Oncologic/Hematologic Diseases: A PRES Subgroup Analysis With a Large Sample Size
- PMID: 34277519
- PMCID: PMC8280768
- DOI: 10.3389/fped.2021.678890
Management and Clinical Outcome of Posterior Reversible Encephalopathy Syndrome in Pediatric Oncologic/Hematologic Diseases: A PRES Subgroup Analysis With a Large Sample Size
Abstract
This study investigated the management and clinical outcomes along with associated factors of posterior reversible encephalopathy syndrome (PRES) in childhood hematologic/oncologic diseases. We present data from children with hematologic/oncologic diseases who developed PRES after treatment of the primary disease with chemotherapy and hematopoietic stem cell transplantation (HSCT) at 3 medical centers in Changsha, China from 2015 to 2020, and review all previously reported cases with the aim of determining whether this neurologic manifestation affects the disease prognosis. In the clinical cohort of 58 PRES patients, hypertension [pooled odds ratio (OR) = 4.941, 95% confidence interval (CI): 1.390, 17.570; P = 0.001] and blood transfusion (OR = 14.259, 95% CI: 3.273, 62.131; P = 0.001) were significantly associated with PRES. Elevated platelet (OR = 0.988, 95% CI: 0.982, 0.995; P < 0.001), hemoglobin (OR = 0.924, 95% CI: 0.890, 0.995; P < 0.001), and blood sodium (OR = 0.905, 95% CI: 0.860, 0.953; P < 0.001), potassium (OR = 0.599, 95% CI: 0.360, 0.995; P = 0.048), and magnesium (OR = 0.093, 95% CI: 0.016, 0.539; P = 0.008) were protective factors against PRES. Data for 440 pediatric PRES patients with hematologic/oncologic diseases in 21 articles retrieved from PubMed, Web of Science, and Embase databases and the 20 PRES patients from our study were analyzed. The median age at presentation was 7.9 years. The most common primary diagnosis was leukemia (62.3%), followed by solid tumor (7.7%) and lymphoma (7.5%). Most patients (65.0%) received chemotherapy, including non-induction (55.2%) and induction (44.8%) regimens; and 86.5% used corticosteroids before the onset of PRES. Although 21.0% of patients died during follow-up, in most cases (93.2%) this was not attributable to PRES but to severe infection (27.3%), underlying disease (26.1%), graft-vs.-host disease (14.8%), multiple organ dysfunction syndrome (8.0%), and respiratory failure (3.4%). PRES was more common with HSCT compared to chemotherapy and had a nearly 2 times higher mortality rate in patients with oncologic/hematologic diseases than in those with other types of disease. Monitoring neurologic signs and symptoms in the former group is therefore critical for ensuring good clinical outcomes following treatment of the primary malignancy.
Keywords: chemotherapy; children; hematopoietic stem cell transplantation; management; neurotoxicity; oncologic/hematologic diseases; posterior reversible encephalopathy syndrome.
Copyright © 2021 Hun, Xie, She, Abdirahman, Li, Wu, Luo, Han, Phorn, Wu, Luo, Chen, Tian, Wan and Wen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


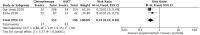

Similar articles
-
Analysis of Risk Factors Associated With Poor Outcome in Posterior Reversible Encephalopathy Syndrome After Treatment in Children: Systematic Review and Meta-Analysis.Front Neurol. 2020 Aug 26;11:938. doi: 10.3389/fneur.2020.00938. eCollection 2020. Front Neurol. 2020. PMID: 32982945 Free PMC article.
-
Posterior Reversible Encephalopathy Syndrome after Allogeneic Stem Cell Transplantation in Pediatric Patients with Fanconi Anemia, a Prospective Study.Biol Blood Marrow Transplant. 2020 Dec;26(12):e316-e321. doi: 10.1016/j.bbmt.2020.08.021. Epub 2020 Aug 27. Biol Blood Marrow Transplant. 2020. PMID: 32860910
-
A survey on hematology-oncology pediatric AIEOP centres: The challenge of posterior reversible encephalopathy syndrome.Eur J Haematol. 2018 Jan;100(1):75-82. doi: 10.1111/ejh.12984. Epub 2017 Nov 9. Eur J Haematol. 2018. PMID: 29032616
-
Posterior reversible encephalopathy syndrome in pediatric hematologic-oncologic disease: literature review and case presentation.Iran J Child Neurol. 2014 Spring;8(2):1-10. Iran J Child Neurol. 2014. PMID: 24949044 Free PMC article. Review.
-
Posterior Reversible Encephalopathy Syndrome in Patients With Cancer.Oncologist. 2015 Jul;20(7):806-11. doi: 10.1634/theoncologist.2014-0149. Epub 2015 Jun 1. Oncologist. 2015. PMID: 26032137 Free PMC article.
Cited by
-
Posterior reversible encephalopathy syndrome in immunocompromised children - A single-center study from South India.J Neurosci Rural Pract. 2024 Apr-Jun;15(2):365-369. doi: 10.25259/JNRP_390_2023. Epub 2024 Feb 13. J Neurosci Rural Pract. 2024. PMID: 38746506 Free PMC article.
-
Magnetic resonance imaging patterns and perfusion changes of posterior reversible encephalopathy syndrome in children with clinical outcome correlation.Pediatr Radiol. 2024 Oct;54(11):1884-1895. doi: 10.1007/s00247-024-06045-w. Epub 2024 Sep 9. Pediatr Radiol. 2024. PMID: 39249148
-
Clinical characteristics and outcomes of children with hypertensive encephalopathy.BMC Pediatr. 2025 Jul 17;25(1):558. doi: 10.1186/s12887-025-05909-w. BMC Pediatr. 2025. PMID: 40676587 Free PMC article.
References
-
- North American Association of Central Cancer Registries (NAACCR) Incidence Data-Cancer in North America (CiNA) Analytic File 1995-2015 [Internet]. Public Use (Which Includes Data From the Center for Disease Control and Prevention's National Program of Cancer Registries [NPCR], the Canadian Council of Cancer Registry's [CCCR's] Provincial and Territorial Registries, and the National Cancer Institute's [NCI's] SEER Registries) (2016).
LinkOut - more resources
Full Text Sources

