Synthesis of New 1 H-1,2,3-Triazole Analogs in Aqueous Medium via " Click" Chemistry: A Novel Class of Potential Carbonic Anhydrase-II Inhibitors
- PMID: 34277561
- PMCID: PMC8278147
- DOI: 10.3389/fchem.2021.642614
Synthesis of New 1 H-1,2,3-Triazole Analogs in Aqueous Medium via " Click" Chemistry: A Novel Class of Potential Carbonic Anhydrase-II Inhibitors
Abstract
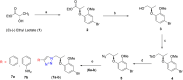
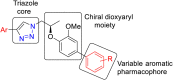
A series of novel 1H-1,2,3-triazole analogs (9a-j) were synthesized via "Click" chemistry and Suzuki-Miyaura cross-coupling reaction in aqueous medium. The compounds were evaluated for their carbonic anhydrase-II enzyme inhibitory activity in vitro. The synthesis of triazole 7a was accomplished using (S)-(-) ethyl lactate as a starting material. This compound (7a) underwent Suzuki-Miyaura cross-coupling reaction with different arylboronic acids in aqueous medium to afford the target molecules, 9a-j in good yields. All newly synthesized compounds were characterized by 1H NMR, 13C NMR, FT-IR, HRMS, and where applicable 19F NMR spectroscopy (9b, 9e, 9h, and 9j). The new compounds have shown moderate inhibition potential against carbonic anhydrase-II enzyme. A preliminary structure-activity relationship suggested that the presence of polar group at the 1H-1,2,3-triazole substituted phenyl ring in these derivatives (9a-j) has contributed to the overall activity of these compounds. Furthermore, via molecular docking, it was deduced that the compounds exhibit inhibitory potential through direct binding with the active site residues of carbonic anhydrase-II enzyme. This study has unraveled a new series of triazole derivatives as good inhibitors against carbonic anhydrase-II.
Keywords: 1H-1, 2, 3-triazole analogs; aqueous medium; carbonic anhydrase-II inhibitory activity; click chemistry; molecular docking studies; synthesis.
Copyright © 2021 Avula, Khan, Halim, Khan, Al-Riyami, Csuk, Das and Al-Harrasi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


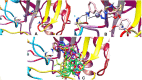

References
-
- Chemical Computing Group ULC. (2014). 1010 Sherbooke St. West, Suite 910. Montreal, QC: Canada. Available at: www.chemcomp.com.
LinkOut - more resources
Full Text Sources

