Formaldehyde Analysis in Non-Aqueous Methanol Solutions by Infrared Spectroscopy and Electrospray Ionization
- PMID: 34277563
- PMCID: PMC8283199
- DOI: 10.3389/fchem.2021.678112
Formaldehyde Analysis in Non-Aqueous Methanol Solutions by Infrared Spectroscopy and Electrospray Ionization
Abstract
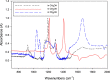
We present the analysis of formaldehyde (HCHO) in anhydrous methanol (CH3OH) as a case study to quantify HCHO in non-aqueous samples. At higher concentrations (C > 0.07 M), we detect a product of HCHO, methoxy methanol (MM, CH3OCH2OH), by Fourier transform infrared spectroscopy, FTIR. Formaldehyde reacts with CH3OH, CD3OH, and CD3OD as shown by FTIR with a characteristic spectral feature around 1,195 cm-1 for CH3OH used for the qualitative detection of MM, a formaldehyde derivative in neat methanol. Ab initio calculations support this assignment. The extinction coefficient for 1,195 cm-1 is in the order of 1.4 × 102 M-1cm-1, which makes the detection limit by FTIR in the order of 0.07 M. For lower concentrations, we performed the quantitative analysis of non-aqueous samples by derivatization with dinitrophenylhydrazine (DNPH). The derivatization uses an aqueous H2SO4 solution to yield the formaldehyde derivatized hydrazone. Ba(OH)2 removes sulfate ions from the derivatized samples and a final extraction with isobutyl acetate to yield a 1:1 methanol: isobutyl acetate solvent for injection for electrospray ionization (ESI). The ESI analysis gave a linear calibration curve for concentrations from 10 to 200 µM with a time-of-flight analyzer (TOF). The detection and quantification limits are 7.8 and 26 μM, respectively, for a linear correlation with R 2 > 0.99. We propose that the formaldehyde in CH3OH is in equilibrium with the MM species, without evidence of HCHO in solution. In the presence of water, the peaks for MM become less resolved, as expected from the well-known equilibria of HCHO that favors the formation of methylene glycol and polymeric species. Our results show that HCHO, in methanol does not exist in the aldehyde form as the main chemical species. Still, HCHO is in equilibrium between the production of MM and the formation of hydrated species in the presence of water. We demonstrate the ESI-MS analysis of HCHO from a non-aqueous TiO2 suspension in methanol. Detection of HCHO after illumination of the colloid indicates that methanol photooxidation yields formaldehyde in equilibrium with the solvent.
Keywords: ESI-TOF; hemiformal; methoxy monoglycol; methoxylated methylene glycol; methoxymetanol.
Copyright © 2021 Barakoti, Subedi, Chalyavi, Gutierrez-Portocarrero, Tucker and Alpuche-Aviles.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Ashdown A., Kletz T. A. (1948). The Infra-red Spectra of Mixtures of Aldehydes and Alcohols. J. Chem. Soc. (Resumed) 296, 1454–1456.
-
- Celik F. E., Lawrence H., Bell A. T. (2008). Synthesis of Precursors to Ethylene Glycol from Formaldehyde and Methyl Formate Catalyzed by Heteropoly Acids. J. Mol. Catal. A: Chem. 288, 87–96. 10.1016/j.molcata.2008.03.029 - DOI
-
- Childers C. L., Huang H., Korzeniewski C. (1999). Formaldehyde Yields from Methanol Electrochemical Oxidation on Carbon-Supported Platinum Catalysts. Langmuir 15, 786–789. 10.1021/la980798o - DOI
LinkOut - more resources
Full Text Sources

