Biomarkers of therapeutic response with immune checkpoint inhibitors
- PMID: 34277840
- PMCID: PMC8267267
- DOI: 10.21037/atm-20-6396
Biomarkers of therapeutic response with immune checkpoint inhibitors
Abstract
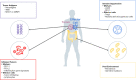
Immune checkpoint inhibitors (ICPIs) have revolutionized the treatment paradigm of a wide range of malignancies with durable responses seen in even advanced, refractory cancers. Unfortunately, only a small proportion of patients with cancer derive meaningful benefit to ICPI therapy, and its use is also limited by significant immune and financial toxicities. Thus, there is a critical need for the development of biomarkers to reliably predict response to ICPI therapy. Only a few biomarkers are validated and approved for use with currently Food and Drug administration (FDA)-approved ICPIs. The development and broad application of biomarkers is limited by the lack of complete understanding of the complex interactions of tumor-host environment, the effect of immunotherapies on these already complex interactions, a lack of standardization and interpretation of biomarker assays across tumor types. Despite these challenges, the field of identifying predictive biomarkers is evolving at an unprecedented pace leaving the clinician responsible for identifying the patients that may derive optimal benefit from ICPIs. In this review, we provide clinicians with a current and practical update on the key, clinically relevant biomarkers of response to ICPIs. We categorize the current and emerging biomarkers of response to ICPIs in four major categories that govern anticancer response-the inflamed tumor, tumor antigens, immune suppression, and overall host environment.
Keywords: Biomarker; immune checkpoint inhibitors (ICPIs); predictive; prognostic.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-6396). The series “Cancer Immunotherapy: Recent Advances and Challenges” was commissioned by the editorial office without any funding or sponsorship. JEG reports grants and personal fees from AstraZeneca, personal fees from Blueprint Medicines, grants from Boehringer Ingelheim, grants and personal fees from Bristol-Myers Squibb, personal fees from EMD Serono - Merck KGaA, grants from Genentech, grants from G 1 Therapeutics, personal fees from Inivata, grants and personal fees from Merck, grants and personal fees from Novartis, grants from Pfizer, grants from Ludwig Institute of Cancer Research, outside the submitted work. SP reports that she is Advisory Board of AstraZeneca, Consultant of G1 therapeutics, and reports Research funding from 5 for the Fight. Dr. TAB reports grants from Bristol Meyers Squibb (BMS), outside the submitted work. The authors have no other conflicts of interest to declare.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
