The dark side of immunotherapy
- PMID: 34277841
- PMCID: PMC8267325
- DOI: 10.21037/atm-20-4750
The dark side of immunotherapy
Abstract
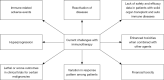
Immunotherapy has broadened the therapeutic scope and response for many cancer patients with drugs that are generally of higher efficacy and less toxicity than prior therapies. Multiple classes of immunotherapies such as targeted antibodies and immune checkpoint inhibitors (ICI), cell-based immunotherapies, immunomodulators, vaccines, and oncolytic viruses have been developed to help the immune system target and destroy malignant tumors. ICI targeting programmed cell death protein-1 (PD-1) or its ligand (PD-L1) are among the most effective immunotherapy agents and are a major focus of current investigations. They have received approval for at least 16 different tumor types as well as for unresectable or metastatic tumors with microsatellite instability-high (MSI-H) or mismatch repair deficiency or with high tumor mutational burden (defined as ≥10 mutations/megabase). However, it is important to recognize that immunotherapy may be associated with significant adverse events. To summarize these events, we conducted a PubMed and Google Scholar database search through April 2020 for manuscripts evaluating treatment-related adverse events and knowledge gaps associated with the use of immunotherapy. Reviewed topics include immune-related adverse events (irAEs), toxicities on combining immunotherapy with other agents, disease reactivation such as tuberculosis (TB) and sarcoid-like granulomatosis, tumor hyperprogression (HPD), financial toxicity, challenges in special patient populations such as solid organ transplant recipients and those with auto-immune diseases. We also reviewed reports of worse or even lethal outcomes compared to other oncologic therapies in certain scenarios and summarized biomarkers predicting adverse events.
Keywords: Immunotherapy; autoimmune; hyperprogression (HPD); immune-related adverse events (irAEs); solid organ transplant.
2021 Annals of Translational Medicine. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4750). The series “Cancer Immunotherapy: Recent Advances and Challenges” was commissioned by the editorial office without any funding or sponsorship. US served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Annals of Translational Medicine from May 2019 to Apr 2021. Dr. SHL reports consulting for Amgen, Merck, Genmab, Xencor, BMS, research support from BMS, Merck, Vaccinex and contracted research from Neon Therapeutics, Astellas, F Star, Xencor, Merck, Vedanta, Boehringer Ingelheim. The authors have no other conflicts of interest to declare.
Figures
References
-
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. Longo DL, editor. N Engl J Med 2018;378:158-68. - PubMed
-
- FDA Approval Timeline of Active Immunotherapies - Cancer Research Institute (CRI) [Internet]. [cited 2020 Jun 15]. Available online: https://www.cancerresearch.org/scientists/immuno-oncology-landscape/fda-...
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous