CryoSIM: super-resolution 3D structured illumination cryogenic fluorescence microscopy for correlated ultrastructural imaging
- PMID: 34277893
- PMCID: PMC8262592
- DOI: 10.1364/OPTICA.393203
CryoSIM: super-resolution 3D structured illumination cryogenic fluorescence microscopy for correlated ultrastructural imaging
Abstract
Rapid cryopreservation of biological specimens is the gold standard for visualizing cellular structures in their true structural context. However, current commercial cryo-fluorescence microscopes are limited to low resolutions. To fill this gap, we have developed cryoSIM, a microscope for 3D super-resolution fluorescence cryo-imaging for correlation with cryo-electron microscopy or cryo-soft X-ray tomography. We provide the full instructions for replicating the instrument mostly from off-the-shelf components and accessible, user-friendly, open-source Python control software. Therefore, cryoSIM democratizes the ability to detect molecules using super-resolution fluorescence imaging of cryopreserved specimens for correlation with their cellular ultrastructure.
Published by The Optical Society under the terms of the Creative Commons Attribution 4.0 License. Further distribution of this work must maintain attribution to the author(s) and the published article’s title, journal citation, and DOI.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

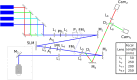


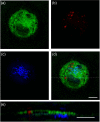
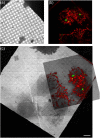
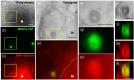
References
-
- Chen B.-C., Legant W. R., Wang K., Shao L., Milkie D. E., Davidson M. W., Janetopoulos C., Wu X. S., Hammer J. A., Liu Z., English B. P., Mimori-Kiyosue Y., Romero D. P., Ritter A. T., Lippincott-Schwartz J., Fritz-Laylin L., Mullins R. D., Mitchell D. M., Bembenek J. N., Reymann A.-C., Böhme R., Grill S. W., Wang J. T., Seydoux G., Tulu U. S., Kiehart D. P., Betzig E., “Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution,” Science 346, 1257998 (2014).SCIEAS10.1126/science.1257998 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
