Enhancing the Separation Performance of Aqueous Phase Separation-Based Membranes through Polyelectrolyte Multilayer Coatings and Interfacial Polymerization
- PMID: 34278307
- PMCID: PMC8276274
- DOI: 10.1021/acsapm.1c00457
Enhancing the Separation Performance of Aqueous Phase Separation-Based Membranes through Polyelectrolyte Multilayer Coatings and Interfacial Polymerization
Abstract
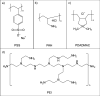
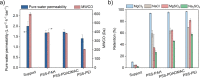
The aqueous phase separation (APS) technique allows membrane fabrication without use of unsustainable organic solvents, while at the same time, it provides extensive control over membrane pore size and morphology. Herein, we investigate if polyelectrolyte complexation-induced APS ultrafiltration membranes can be the basis for different types of nanofiltration membranes. We demonstrate that APS membranes can be used as support membranes for functional surface coatings like thin polyelectrolyte multilayer (PEMs) and interfacial polymerization (IP) coatings. Three different PEMs were fabricated on poly(sodium 4-styrene sulfonate) (PSS) poly(allylamine hydrochloride) (PAH) APS ultrafiltration membranes, and only 4.5 bilayers were needed to create nanofiltration membranes with molecular weight cut-off (MWCO) values of 210-390 Da while maintaining a roughly constant water permeability (∼1.7 L·m-2·h-1·bar-1). The PEM-coated membranes showed excellent MgCl2 (∼98%), NaCl (∼70%), and organic micropollutant retention values (>90%). Similarly, fabricating thin polyamide layers on the ultrafiltration PSS-PAH APS membranes by IP resulted in nanofiltration membranes with MWCO values of ∼200 Da. This work shows for the first time that APS membranes can indeed be utilized as excellent support membranes for the application of functional coatings without requiring any form of pretreatment.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Nunes S. P.; Culfaz-Emecen P. Z.; Ramon G. Z.; Visser T.; Koops G. H.; Jin W.; Ulbricht M. Thinking the Future of Membranes: Perspectives for Advanced and New Membrane Materials and Manufacturing Processes. J. Membr. Sci. 2020, 598, 117761.10.1016/j.memsci.2019.117761. - DOI
-
- Kim D.; Nunes S. P. Green Solvents for Membrane Manufacture: Recent Trends and Perspectives. Curr. Opin. Green Sustain. Chem. 2021, 28, 100427.10.1016/j.cogsc.2020.100427. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

