Incident Cases of Sexually Transmitted Infections among Users of Pre-Exposure Prophylaxis for HIV Prevention in Honolulu, Hawai'i
- PMID: 34278321
- PMCID: PMC8280359
Incident Cases of Sexually Transmitted Infections among Users of Pre-Exposure Prophylaxis for HIV Prevention in Honolulu, Hawai'i
Abstract
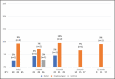
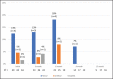
Emtricitabine/tenofovir disoproxil fumarate [FTC-TDF] is a daily oral medication taken by HIV-negative individuals for pre-exposure prophylaxis (PrEP) to prevent human immunodeficiency virus (HIV) infection. A higher incidence of sexually transmitted infections (STIs) among PrEP users has been reported compared to STI incidence before PrEP use. Asymptomatic incident STI rates were investigated among 78 patients presenting for PrEP in Honolulu, Hawai'i, from April 2018 to May 2019. Testing for oropharyngeal gonorrhea, urethral gonorrhea and chlamydia, rectal gonorrhea and chlamydia, and syphilis was performed. Incident STI percentages were calculated at each follow-up visit. Ninety-seven percent of patients were men who have sex with men (MSM). Forty-seven percent of patients had follow-up data 6 months after initiation and 28% after 1 year. Thirty-two percent of patients self-reported an STI before initiating PrEP. More than half reported anonymous partners. There were 35 positive STI tests during the study period, and 25% of patients had one or more positive tests during this time. At initiation, 17% of patients were found to have an STI, followed by 16% at 3 months, 14% at 6 months, 8% at 9 months, and 5% at 12 months. At all visits, chlamydia was the most common STI detected; at 6 months, 18% of all rectal tests were positive for chlamydia. There were inconsistent condom use and high STI rates from screening during PrEP initiation and follow-up, offering an opportunity to identify asymptomatic STIs in this population. This study is the first report in Hawai'i of STI rates among PrEP users.
Keywords: HIV prevention; Pre-Exposure Prophylaxis; Sexually transmitted infections.
©Copyright 2021 by University Health Partners of Hawai‘i (UHP Hawai‘i).
Figures
Similar articles
-
Sexually Transmitted Infection Screening Among Gay, Bisexual, and Other Men Who Have Sex With Men Prescribed Pre-exposure Prophylaxis in Baltimore City, Maryland.Clin Infect Dis. 2020 Dec 17;71(10):2637-2644. doi: 10.1093/cid/ciz1145. Clin Infect Dis. 2020. PMID: 31761944
-
Low incidence of HIV infection and decreasing incidence of sexually transmitted infections among PrEP users in 2020 in Germany.Infection. 2023 Jun;51(3):665-678. doi: 10.1007/s15010-022-01919-3. Epub 2022 Sep 27. Infection. 2023. PMID: 36168098 Free PMC article.
-
HIV pre-exposure prophylaxis and diagnoses of sexually transmitted infections - observational data from German checkpoints, 01/2019-08/2021.BMC Public Health. 2023 Apr 7;23(1):661. doi: 10.1186/s12889-023-15570-6. BMC Public Health. 2023. PMID: 37029369 Free PMC article.
-
Global Epidemiologic Characteristics of Sexually Transmitted Infections Among Individuals Using Preexposure Prophylaxis for the Prevention of HIV Infection: A Systematic Review and Meta-analysis.JAMA Netw Open. 2019 Dec 2;2(12):e1917134. doi: 10.1001/jamanetworkopen.2019.17134. JAMA Netw Open. 2019. PMID: 31825501 Free PMC article.
-
The impact of HIV preexposure prophylaxis on bacterial sexually transmitted infection occurrence in MSM: a systematic review and meta-analysis.AIDS. 2024 Jun 1;38(7):1033-1045. doi: 10.1097/QAD.0000000000003837. Epub 2024 Jan 11. AIDS. 2024. PMID: 38669203
References
-
- Centers for Disease Control and Prevention US Public Health Service Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update A Clinical Practice Guideline. 2018. Mar, Available from: https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf. Accessed October 24, 2019.
-
- Food and Drug Administration Truvada for PrEP Fact Sheet: Ensuring Safe and Proper Use. 2012. Available from: https://www.fda.gov/media/83586/download. Accessed March 13, 2021.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

