Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany
- PMID: 34278371
- PMCID: PMC8278033
- DOI: 10.1016/j.lanepe.2021.100164
Emergence of the E484K mutation in SARS-COV-2-infected immunocompromised patients treated with bamlanivimab in Germany
Abstract
Background: Monoclonal antibodies (mAb) have been introduced as a promising new therapeutic approach against SARS-CoV-2. At present, there is little experience regarding their clinical effects in patient populations underrepresented in clinical trials, e.g. immunocompromised patients. Additionally, it is not well known to what extent SARS-CoV-2 treatment with monoclonal antibodies could trigger the selection of immune escape viral variants.
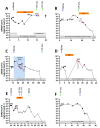
Methods: After identifying immunocompromised patients with viral rebound under treatment with bamlanivimab, we characterized the SARS-CoV-2-isolates by whole genome sequencing. Viral load measurements and sequence analysis were performed consecutively before and after bamlanivimab administration.
Findings: After initial decrease of viral load, viral clearance was not achieved in five of six immunocompromised patients treated with bamlanivimab. Instead, viral replication increased again over the course of the following one to two weeks. In these five patients, the E484K substitution - known to confer immune escape - was detected at the time of viral rebound but not before bamlanivimab treatment.
Interpretation: Treatment of SARS-CoV-2 with bamlanivimab in immunocompromised patients results in the rapid development of immune escape variants in a significant proportion of cases. Given that the E484K mutation can hamper natural immunity, the effectiveness of vaccination as well as antibody-based therapies, these findings may have important implications not only for individual treatment decisions but may also pose a risk to general prevention and treatment strategies.
Funding: All authors are employed and all expenses covered by governmental, federal state, or other publicly funded institutions.
© 2021 The Author(s).
Conflict of interest statement
BJ received honoraria for presentations from Gilead (Remdesivir) as well as Falk, Janssen-Cilag, MSD, BMS, Abbvie, ViiV, Gilead, Boehringer, Fresenius Medical Care (outside the submitted work) and served on advisory boards for ViiV, BMS, Gilead, Theratechnologies (outside the submitted work). VK received lecture fees from Abbvie, Falk, Albireo, Gilead (outside the submitted work). TF was PI for a Gilead clinical trial (Remdesivir) and served on Gilead advisory boards. All other authors declare no competing interests regarding this work.
Figures
References
-
- Pallotta A.M., Kim C., Gordon S.M., Kim A. Monoclonal antibodies for treating COVID-19. Cleve Clin J Med. 2021 - PubMed
-
- Collier D.A., De Marco A., Ferreira I. SARS-CoV-2 B1.1.7 sensitivity to mRNA vaccine-elicited, convalescent and monoclonal antibodies. medRxiv. 2021
LinkOut - more resources
Full Text Sources
Miscellaneous

