Recent advances in palladium-catalyzed (hetero)annulation of C[double bond, length as m-dash]C bonds with ambiphilic organo(pseudo)halides
- PMID: 34278397
- PMCID: PMC8364377
- DOI: 10.1039/d1cc02836g
Recent advances in palladium-catalyzed (hetero)annulation of C[double bond, length as m-dash]C bonds with ambiphilic organo(pseudo)halides
Abstract
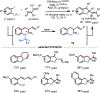
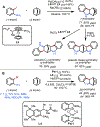
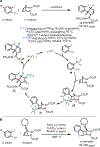
Palladium has proven to be effective in catalyzing the (hetero)annulation of C[double bond, length as m-dash]C bonds with ambiphilic organo(pseudo)halides. Through the employment of appropriate ambiphilic coupling partners, efficient annulation of a variety of allenes, 1,3-dienes, strained alkenes, styrenes, and other C[double bond, length as m-dash]C bond variants can be achieved to provide direct access to numerous useful hetero- and carbocyclic scaffolds. In this Feature Article, we summarize palladium-catalyzed (hetero)annulation methods reported since 2005 (spanning just over 15 years) and discuss outstanding challenges in this area of study.
Conflict of interest statement
Conflicts of interest
In accordance with our policy on Conflicts of interest please ensure that a conflicts of interest statement is included in your manuscript here. Please note that this statement is required for all submitted manuscripts. If no conflicts exist, please state that “There are no conflicts to declare”.
Figures



































References
-
-
For selected reviews, see: Liu D, Zhao Gand Xiang L, Eur. J. Org. Chem, 2010, 2010, 3975;Silva TS, Rodrigues MT Jr., Santos H, Zeoly LA, Almeida WP, Barcelos RC, Gomes RC, Fernandes FS and Coelho F, Tetrahedron, 2019, 75, 2063;Bertolini F and Pineschi M, Org. Prep. Proc. Int, 2009, 41, 385;Garlets ZJ, White DR and Wolfe JP, Asian J. Org. Chem, 2017, 6, 636.
-
-
- Larock RC, Harrison LW and Hsu MH, J. Org. Chem, 1984, 49, 3662;
- Larock RC and Song H, Synth. Commun, 1989, 19, 1463;
- Larock RC, Varaprath S, Lau HH and Fellows CA, J. Am. Chem. Soc, 1984, 106, 5274;
- Larock RC, Liu C-L, Lau HH and Varaprath S, Tetrahedron Lett, 1984, 25, 4459.
-
- O’Connor JM, Stallman BJ, Clark WG, Shu AYL, Spada RE, Stevenson TM and Dieck HA, J. Org. Chem, 1983, 48, 807.
-
- Larock RC and Fried CA, J. Am. Chem. Soc, 1990, 112, 5882;
- Larock RC, Berrios-Peña N and Narayanan K, J. Org. Chem, 1990, 55, 3447.
-
- Herraiz-Cobo J, Albericio F and Álvarez M, in Advances in Heterocyclic Chemistry, ed. Scriven EFV and Ramsden CA, Academic Press, USA, 1st edn, 2015, vol. 116, ch. 1, pp. 1–35.
Grants and funding
LinkOut - more resources
Full Text Sources

